Cetuximab is efficient and safe in patients with advanced cutaneous squamous cell carcinoma: a retrospective, multicentre study
- PMID: 32064041
- PMCID: PMC6996917
- DOI: 10.18632/oncotarget.27434
Cetuximab is efficient and safe in patients with advanced cutaneous squamous cell carcinoma: a retrospective, multicentre study
Abstract
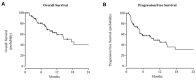
There is no standard of care for unresectable cutaneous squamous cell carcinoma (cSCC). Chemotherapy, alone or combined with radiotherapy, is commonly used mostly as palliative treatment; moreover, its poor safety profile limits its use most of the time, especially in elderly patients. Thus, alternative options are needed. Targeted molecular inhibitors, such as the epidermal growth factor receptor inhibitor cetuximab, seem promising, but data are limited. We retrospectively evaluated clinical outcomes of cetuximab as a single agent in this indication. The primary endpoint was the Disease Control Rate (DCR) at 6 weeks according to RECIST criteria. Secondary endpoints included DCR at 12 weeks, objective response rate (ORR) at 6 and 12 weeks, progression-free-survival (PFS), overall survival (OS), and safety profile. Fifty-eight patients received cetuximab as monotherapy. The median age was 83.2 (range, 47.4 to 96.1). The majority of patients was chemotherapy naïve. The median follow-up was 11.7 months (95% CI: 9.6-30.1). The DCR at 6 and 12 weeks was 87% and 70%, respectively. The ORR was 53% and 42%, respectively, at 6 and 12 weeks. The median PFS and OS were 9.7 months (95% CI: 4.8-43.4) and 17.5 months (95% CI: 9.4-43.1), respectively. Fifty-one patients (88%) experienced toxicity, and 67 adverse events related to cetuximab occurred. Most of them (84%) were grade 1 to 2. Our study shows that cetuximab is safe and efficient for the treatment of patients, even elderly ones, with advanced cSCC. These results indicate that cetuximab is a promising agent to test in new combinations, especially with immune checkpoint inhibitors such as anti-PD-1 agents.
Keywords: cetuximab; cutaneous squamous cell carcinoma; epidermal growth factor receptor.
Copyright: Montaudié et al.
Conflict of interest statement
CONFLICTS OF INTEREST FP is a Merck board member. HM is principal investigator in studies conducted by BMS, MSD. Research grant to institution received BMS, LeoPharma. SD is principal investigator in studies conducted by BMS, MSD, Merck, Regeneron. Travel grants covered by BMS. Research grant to institution received from BMS, MSD.
Figures
References
-
- Stratigos A, Garbe C, Lebbe C, Malvehy J, del Marmol V, Pehamberger H, Peris K, Becker JC, Zalaudek I, Saiag P, Middleton MR, Bastholt L, Testori A, Grob JJ, and European Dermatology Forum (EDF), and European Association of Dermato-Oncology (EADO), and European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015; 51:1989–2007. 10.1016/j.ejca.2015.06.110. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials