Nimotuzumab-cisplatin-radiation versus cisplatin-radiation in HPV negative oropharyngeal cancer
- PMID: 32064043
- PMCID: PMC6996911
- DOI: 10.18632/oncotarget.27443
Nimotuzumab-cisplatin-radiation versus cisplatin-radiation in HPV negative oropharyngeal cancer
Abstract
Background: Addition of nimotuzumab to weekly cisplatin and radiation improves outcomes in head and neck cancer. HPV negative oropharyngeal cancer has unsatisfactory treatment outcomes and is a candidate for escalation of treatment. We wanted to determine whether the addition of nimotuzumab to cisplatin-radiation could improve outcomes in these poor-risk tumors.
Methods: This was a subgroup analysis of a phase 3 randomized study. In this study, locally advanced head and neck cancer patients undergoing definitive chemoradiation were randomly allocated to weekly cisplatin (30 mg/m2 IV)- radiation (66-70 Gy) {CRT arm} or nimotuzumab (200 mg weekly) -weekly cisplatin (30 mg/m2)-radiation (66-70 Gy) {NCRT arm}. The data of HPV negative oropharyngeal cancer was extracted from the database of this study for the analysis. HPV testing was done with p16 immunohistochemistry (IHC) staining and reported according to the CAP criteria. The outcomes assessed were progression-free survival (PFS), disease-free survival (DFS), locoregional control, and overall survival (OS). Interaction test was performed between the study arms and HPV status prior to doing any HPV specific analysis for each of the studied outcomes. Kaplan Meier estimates for 2 year OS with 95%CI was calculated. The hazard ratio was obtained using COX regression analysis.
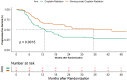
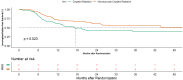
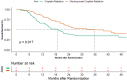
Results: We had 187 HPV negative oropharyngeal cancers, 91 in the CRT arm and 96 in NCRT arm. The interaction test was significant for PFS (p = 0.000), locoregional control (p = 0.007) and overall survival (p = 0.002) but not for DFS (p = 0.072). The 2- year PFS was 31.5% (95%CI 21.5-42) in CRT arm versus 57.2% (95%CI 45.8-67.1) in NCRT arm (HR -0.54; 95%CI 0.36-0.79, p = 0.002). The 2-year LRC was 41.4% (95%CI 29.8-52.6) in the CRT arm versus in 60.4% (95%CI 48.7-70.2) in the NCRT arm (HR -0.61; 95%CI 0.4-0.94, p = 0.024). The addition of nimotuzumab also lead to an improvement in 2-year OS from 39.0% (95%CI 28.4-49.6) to 57.6% (95%CI 46.3-67.4) (HR-0.63, 95%CI 0.43-0.92, p = 0.018).
Conclusions: The addition of nimotuzumab to weekly cisplatin-radiation improves outcomes inclusive of OS in HPV negative oropharyngeal cancers.
Keywords: HPV negative; cisplatin; nimotuzumab; oropharynx; weekly.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. Dr. Noronha reports research grants from Dr. Reddy’s Laboratories Inc, Amgen, Sanofi India Ltd., Intas Pharmaceuticals and Astra Zeneca Pharma India Ltd., outside the submitted work. Dr. Prabhash reports grants from Biocon Ltd, grants from Dr. Reddy’s Laboratories Inc, grants from Fresenius Kabi India Pvt Ltd, grants from Alkem Laboratories, grants from Natco Pharma Ltd, grants from BDR Pharmaceuticals Intl Pvt Ltd, grants from Roche Holding AG, outside the submitted work. All grants were paid to the institution. None of the other authors have anything to declare that may be considered as potential competing interests.
Figures
References
-
- Murthy V, Calcuttawala A, Chadha K, d’Cruz A, Krishnamurthy A, Mallick I, Nair S, Teni T, Pawar S, Talapatra K, Patil A, Bhatt A, Chatterjee S, et al. Human papillomavirus in head and neck cancer in India: current status and consensus recommendations. South Asian J Cancer. 2017; 6:93–98. 10.4103/sajc.sajc_96_17. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

