A comparative study of nivolumab and axitinib in terms of overall survival, treatment continuation, and cost for patients with metastatic renal cell carcinoma
- PMID: 32064108
- PMCID: PMC7016520
- DOI: 10.3892/mco.2020.1978
A comparative study of nivolumab and axitinib in terms of overall survival, treatment continuation, and cost for patients with metastatic renal cell carcinoma
Abstract
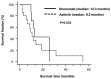
Nivolumab and axitinib has recommended as a second-line treatment in patients with metastatic renal cell carcinoma (mRCC) after tyrosine kinase inhibitor treatment. In this study, overall survival (OS), treatment continuation, and the cost of nivolumab and axitinib-the second-line treatment agents for mRCC-were compared and examined. Herein, we retrospectively surveyed patients with pathologically confirmed mRCC, treated with nivolumab (n=9) or axitinib (n=16) at Ogaki Municipal Hospital (Ogaki, Japan) between January 2012 and May 2019. The treatment periods for the nivolumab- and axitinib-administered groups were 5.4 (range: 1.4-21.3) and 3.4 (range: 0.3-28.1) months, respectively (P=0.089). The postponement periods for the nivolumab- and axitinib-administered groups were 7 (range: 0-186) and 0 (range: 0-262) days, respectively, and the difference was statistically significant (P=0.008). The median OS for patients treated with nivolumab and axitinib was 12.3 (range: 1.5-25.5 months) and 9.2 (range: 2.2-55.0 months) months, respectively (P=0.633). The one-year cost estimates for axitinib and nivolumab in clinical practice were $60,694.2 and $86,544.4, respectively (P=0.017). We found that despite frequent interruptions in nivolumab administration and a longer postpaonement period for the nivolumab-administered group than for the axitinib-administered group, both groups exhibit comparable treatment duration and OS.
Keywords: advanced/recurrent renal cancer; axitinib; nivolumab; overall survival; second-line treatment.
Copyright © 2019, Spandidos Publications.
Figures
References
-
- Wang C, Thudium KB, Han M, Wang XT, Huang H, Feingersh D, Garcia C, Wu Y, Kuhne M, Srinivasan M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2:846–856. doi: 10.1158/2326-6066.CIR-14-0040. - DOI - PubMed
LinkOut - more resources
Full Text Sources