Unraveling virus relationships by structure-based phylogenetic classification
- PMID: 32064119
- PMCID: PMC7015158
- DOI: 10.1093/ve/veaa003
Unraveling virus relationships by structure-based phylogenetic classification
Abstract
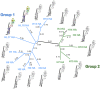
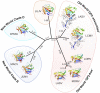
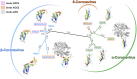
Delineation of the intricacies of protein function from macromolecular structure constitutes a continual obstacle in the study of cell and pathogen biology. Structure-based phylogenetic analysis has emerged as a powerful tool for addressing this challenge, allowing the detection and quantification of conserved architectural properties between proteins, including those with low or no detectable sequence homology. With a focus on viral protein structure, we highlight how a number of investigations have utilized this powerful method to infer common functionality and ancestry.
Keywords: evolution; function; protein; structure; virus.
© The Author(s) 2020. Published by Oxford University Press.
Figures


References
-
- Abrescia N. G. et al. (2008) ‘Insights into Virus Evolution and Membrane Biogenesis from the Structure of the Marine Lipid-Containing Bacteriophage PM2’, Molecular Cell, 31: 749–61. - PubMed
-
- Abrescia N. G. et al. (2012) ‘Structure Unifies the Viral Universe’, Annual Review of Biochemistry, 81: 795–822. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

