Changes in Intestinal Microbiota Are Associated with Islet Function in a Mouse Model of Dietary Vitamin A Deficiency
- PMID: 32064275
- PMCID: PMC6996671
- DOI: 10.1155/2020/2354108
Changes in Intestinal Microbiota Are Associated with Islet Function in a Mouse Model of Dietary Vitamin A Deficiency
Abstract
Aims: The underlying mechanisms involved in Vitamin A- (VA-) related changes in glucose metabolic disorders remain unclear. Recent evidence suggests that intestinal microbiota is closely linked to the metabolic syndrome. Here, we explored whether and how intestinal microbiota affects glucose homeostasis in VA-deficient diet-fed mice.
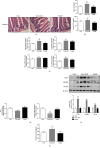
Methods: Six-week-old male C57BL/6 mice were randomly placed on either a VA-sufficient (VAS) or VA-deficient (VAD) diet for 10 weeks. Subsequently, a subclass of the VAD diet-fed mice was switched to a VA-deficient rescued (VADR) diet for an additional 8 weeks. The glucose metabolic phenotypes of the mice were assessed using glucose tolerance tests and immunohistochemistry staining. Changes in intestinal microbiota were assessed using 16S gene sequencing. The intestinal morphology, intestinal permeability, and inflammatory response activation signaling pathway were assessed using histological staining, western blots, quantitative-PCR, and enzyme-linked immunosorbent assays.
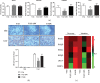
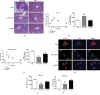
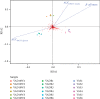
Results: VAD diet-fed mice displayed reduction of tissue VA levels, increased area under the curve (AUC) of glucose challenge, reduced glucose-stimulated insulin secretion, and loss of β cell mass. Redundancy analysis showed intestinal microbiota diversity was significantly associated with AUC of glucose challenge and β cell mass. Redundancy analysis showed intestinal microbiota diversity was significantly associated with AUC of glucose challenge and κB signaling pathway activation. Reintroduction of dietary VA to VAD diet-fed mice restored tissue VA levels, endocrine hormone profiles, and inflammatory response, which are similar to those observed following VAS-controlled changes in intestinal microbiota.
Conclusions: We found intestinal microbiota effect islet function via controlling intestinal inflammatory phenotype in VAD diet-fed mice. Intestinal microbiota influences could be considered as an additional mechanism for the effect of endocrine function in a VAD diet-driven mouse model.
Copyright © 2020 Yunting Zhou et al.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

Similar articles
-
Vitamin A deficiency causes islet dysfunction by inducing islet stellate cell activation via cellular retinol binding protein 1.Int J Biol Sci. 2020 Jan 30;16(6):947-956. doi: 10.7150/ijbs.37861. eCollection 2020. Int J Biol Sci. 2020. PMID: 32140064 Free PMC article.
-
Maternal Vitamin A deficiency during pregnancy and lactation induced damaged intestinal structure and intestinal flora homeostasis in offspring mice.Food Sci Nutr. 2023 Apr 12;11(6):3422-3432. doi: 10.1002/fsn3.3332. eCollection 2023 Jun. Food Sci Nutr. 2023. PMID: 37324834 Free PMC article.
-
Vitamin A influences the incretin hormone profiles by activating the retinoic acid receptor β.J Diabetes Complications. 2024 Aug;38(8):108806. doi: 10.1016/j.jdiacomp.2024.108806. Epub 2024 Jul 8. J Diabetes Complications. 2024. PMID: 38996583
-
Immune Impairment Associated with Vitamin A Deficiency: Insights from Clinical Studies and Animal Model Research.Nutrients. 2022 Nov 26;14(23):5038. doi: 10.3390/nu14235038. Nutrients. 2022. PMID: 36501067 Free PMC article. Review.
-
Mechanisms and consequences of intestinal dysbiosis.Cell Mol Life Sci. 2017 Aug;74(16):2959-2977. doi: 10.1007/s00018-017-2509-x. Epub 2017 Mar 28. Cell Mol Life Sci. 2017. PMID: 28352996 Free PMC article. Review.
Cited by
-
Role and Mechanism of Vitamin A Metabolism in the Pathophysiology of Parkinson's Disease.J Parkinsons Dis. 2021;11(3):949-970. doi: 10.3233/JPD-212671. J Parkinsons Dis. 2021. PMID: 34120916 Free PMC article. Review.
-
Vitamin A Status Improvement in Obesity: Findings and Perspectives Using Encapsulation Techniques.Nutrients. 2021 Jun 3;13(6):1921. doi: 10.3390/nu13061921. Nutrients. 2021. PMID: 34204998 Free PMC article. Review.
-
The role of gut microbiome and its metabolites in pancreatitis.mSystems. 2024 Oct 22;9(10):e0066524. doi: 10.1128/msystems.00665-24. Epub 2024 Aug 30. mSystems. 2024. PMID: 39212377 Free PMC article. Review.
-
Time-dependent changes in retinoids content in liver and adipose tissue after feeding of a vitamin A-deficient diet to mice.Exp Anim. 2024 Jul 9;73(3):302-309. doi: 10.1538/expanim.23-0123. Epub 2024 Feb 21. Exp Anim. 2024. PMID: 38382988 Free PMC article.
-
The Pathogenesis of Diabetes.Int J Mol Sci. 2023 Apr 10;24(8):6978. doi: 10.3390/ijms24086978. Int J Mol Sci. 2023. PMID: 37108143 Free PMC article. Review.
References
-
- WHO. WHO global database on vitamin A deficiency. Vol. 2009. Geneva: WHO Library Cataloguing-in-Publication Data; Global prevalence of vitamin A deficiency in populations at risk 1995-2005.
-
- Reddy M. R. G., Venkata S. M., Putcha U. K., Jeyakumar S. M. Vitamin A deficiency induces endoplasmic reticulum stress and apoptosis in pancreatic islet cells: implications of stearoyl-CoA desaturase 1-mediated oleic acid synthesis. Experimental Cell Research. 2018;364(1):104–112. doi: 10.1016/j.yexcr.2018.01.040. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

