Poly(amino acids) as a potent self-adjuvanting delivery system for peptide-based nanovaccines
- PMID: 32064333
- PMCID: PMC6989150
- DOI: 10.1126/sciadv.aax2285
Poly(amino acids) as a potent self-adjuvanting delivery system for peptide-based nanovaccines
Abstract
To be optimally effective, peptide-based vaccines need to be administered with adjuvants. Many currently available adjuvants are toxic, not biodegradable; they invariably invoke adverse reactions, including allergic responses and excessive inflammation. A nontoxic, biodegradable, biocompatible, self-adjuvanting vaccine delivery system is urgently needed. Herein, we report a potent vaccine delivery system fulfilling the above requirements. A peptide antigen was coupled with poly-hydrophobic amino acid sequences serving as self-adjuvanting moieties using solid-phase synthesis, to produce fully defined single molecular entities. Under aqueous conditions, these molecules self-assembled into distinct nanoparticles and chain-like aggregates. Following subcutaneous immunization in mice, these particles successfully induced opsonic epitope-specific antibodies without the need of external adjuvant. Mice immunized with entities bearing 15 leucine residues were able to clear bacterial load from target organs without triggering the release of soluble inflammatory mediators. Thus, we have developed a well-defined and effective self-adjuvanting delivery system for peptide antigens.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures
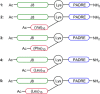
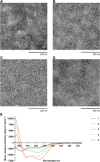
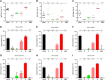
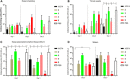
References
-
- Purcell A. W., McCluskey J., Rossjohn J., More than one reason to rethink the use of peptides in vaccine design. Nat. Rev. Drug Discov. 6, 404–414 (2007). - PubMed
-
- Melero I., Gaudernack G., Gerritsen W., Huber C., Parmiani G., Scholl S., Thatcher N., Wagstaff J., Zielinski C., Faulkner I., Mellstedt H., Therapeutic vaccines for cancer: An overview of clinical trials. Nat. Rev. Clin. Oncol. 11, 509–524 (2014). - PubMed
-
- Steer A. C., Carapetis J. R., Dale J. B., Fraser J. D., Good M. F., Guilherme L., Moreland N. J., Mulholland E. K., Schodel F., Smeesters P. R., Status of research and development of vaccines for Streptococcus pyogenes. Vaccine 34, 2953–2958 (2016). - PubMed
-
- Carapetis J. R., Steer A. C., Mulholland E. K., Weber M., The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5, 685–694 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

