Single-cell analysis reveals different age-related somatic mutation profiles between stem and differentiated cells in human liver
- PMID: 32064334
- PMCID: PMC6994209
- DOI: 10.1126/sciadv.aax2659
Single-cell analysis reveals different age-related somatic mutation profiles between stem and differentiated cells in human liver
Erratum in
-
An Erratum for the Research Article: "Single-cell analysis reveals different age-related somatic mutation profiles between stem and differentiated cells in human liver" by K. Brazhnik, S. Sun, O. Alani, M. Kinkhabwala, A. W. Wolkoff, A. Y. Maslov, X. Dong, and J. Vijg.Sci Adv. 2020 Oct 2;6(40):eabe8055. doi: 10.1126/sciadv.abe8055. Print 2020 Oct. Sci Adv. 2020. PMID: 33008900 Free PMC article. No abstract available.
Abstract
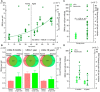
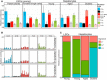
Accumulating somatic mutations have been implicated in age-related cellular degeneration and death. Because of their random nature and low abundance, somatic mutations are difficult to detect except in single cells or clonal cell lineages. Here, we show that in single hepatocytes from human liver, an organ exposed to high levels of genotoxic stress, somatic mutation frequencies are high and increase substantially with age. Considerably lower mutation frequencies were observed in liver stem cells (LSCs) and organoids derived from them. Mutational spectra in hepatocytes showed signatures of oxidative stress that were different in old age and in LSCs. A considerable number of mutations were found in functional parts of the liver genome, suggesting that somatic mutagenesis could causally contribute to the age-related functional decline and increased incidence of disease of human liver. These results underscore the importance of stem cells in maintaining genome sequence integrity in aging somatic tissues.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

