Soil organic matter attenuates the efficacy of flavonoid-based plant-microbe communication
- PMID: 32064339
- PMCID: PMC6989149
- DOI: 10.1126/sciadv.aax8254
Soil organic matter attenuates the efficacy of flavonoid-based plant-microbe communication
Abstract
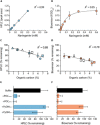
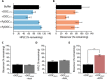
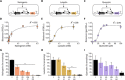
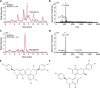
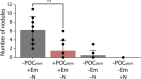
Plant-microbe interactions are mediated by signaling compounds that control vital plant functions, such as nodulation, defense, and allelopathy. While interruption of signaling is typically attributed to biological processes, potential abiotic controls remain less studied. Here, we show that higher organic carbon (OC) contents in soils repress flavonoid signals by up to 70%. Furthermore, the magnitude of repression is differentially dependent on the chemical structure of the signaling molecule, the availability of metal ions, and the source of the plant-derived OC. Up to 63% of the signaling repression occurs between dissolved OC and flavonoids rather than through flavonoid sorption to particulate OC. In plant experiments, OC interrupts the signaling between a legume and a nitrogen-fixing microbial symbiont, resulting in a 75% decrease in nodule formation. Our results suggest that soil OC decreases the lifetime of flavonoids underlying plant-microbe interactions.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures

References
-
- Cesco S., Mimmo T., Tonon G., Tomasi N., Pinton R., Terzano R., Neumann G., Weisskopf L., Renella G., Landi L., Nannipieri P., Plant-borne flavonoids released into the rhizosphere: Impact on soil bio-activities related to plant nutrition. A review. Biol. Fertil. Soils 48, 123–149 (2012).
-
- Mus F., Crook M. B., Garcia K., Garcia Costas A., Geddes B. A., Kouri E. D., Paramasivan P., Ryu M.-H., Oldroyd G. E. D., Poole P. S., Udvardi M. K., Voigt C. A., Ané J.-M., Peters J. W., Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 82, 3698–3710 (2016). - PMC - PubMed
-
- Ahuja I., Kissen R., Bones A. M., Phytoalexins in defense against pathogens. Trends Plant Sci. 17, 73–90 (2012). - PubMed
-
- Khan Z. R., Pickett J. A., Wadhams L. J., Hassanali A., Midega C. A. O., Combined control of Striga hermonthica and stemborers by maize–Desmodium spp. intercrops. Crop Prot. 25, 989–995 (2006).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

