Modified cyclodextrins as broad-spectrum antivirals
- PMID: 32064341
- PMCID: PMC6989148
- DOI: 10.1126/sciadv.aax9318
Modified cyclodextrins as broad-spectrum antivirals
Abstract
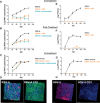
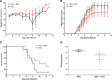
Viral infections kill millions of people and new antivirals are needed. Nontoxic drugs that irreversibly inhibit viruses (virucidal) are postulated to be ideal. Unfortunately, all virucidal molecules described to date are cytotoxic. We recently developed nontoxic, broad-spectrum virucidal gold nanoparticles. Here, we develop further the concept and describe cyclodextrins, modified with mercaptoundecane sulfonic acids, to mimic heparan sulfates and to provide the key nontoxic virucidal action. We show that the resulting macromolecules are broad-spectrum, biocompatible, and virucidal at micromolar concentrations in vitro against many viruses [including herpes simplex virus (HSV), respiratory syncytial virus (RSV), dengue virus, and Zika virus]. They are effective ex vivo against both laboratory and clinical strains of RSV and HSV-2 in respiratory and vaginal tissue culture models, respectively. Additionally, they are effective when administrated in mice before intravaginal HSV-2 inoculation. Lastly, they pass a mutation resistance test that the currently available anti-HSV drug (acyclovir) fails.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- De Clercq E., Strategies in the design of antiviral drugs. Nat. Rev. Drug Discov. 1, 13–25 (2002). - PubMed
-
- McCormack S., Ramjee G., Kamali A., Rees H., Crook A. M., Gafos M., Jentsch U., Pool R., Chisembele M., Kapiga S., Mutemwa R., Vallely A., Palanee T., Sookrajh Y., Lacey C. J., Darbyshire J., Grosskurth H., Profy A., Nunn A., Hayes R., Weber J., PRO2000 vaginal gel for prevention of hiv-1 infection (microbicides development programme 301): A phase 3, randomised, double-blind, parallel-group trial. Lancet 376, 1329–1337 (2010). - PMC - PubMed
-
- Pirrone V., Wigdahl B., Krebs F. C., The rise and fall of polyanionic inhibitors of the human immunodeficiency virus type 1. Antivir. Res. 90, 168–182 (2011). - PubMed
-
- Van Damme L., Govinden R., Mirembe F. M., Guédou F., Solomon S., Becker M. L., Pradeep B., Krishnan A., Alary M., Pande B., Ramjee G., Deese J., Crucitti T., Taylor D.; CS Study Group , Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal hiv transmission. N. Engl. J. Med. 359, 463–472 (2008). - PubMed
-
- Rusnati M., Vicenzi E., Donalisio M., Oreste P., Landolfo S., Lembo D., Sulfated K5 Escherichia coli polysaccharide derivatives: A novel class of candidate antiviral microbicides. Pharmacol. Ther. 123, 310–322 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

