Toxicological Risks of Renqingchangjue in Rats Evaluated by 1H NMR-Based Serum and Urine Metabolomics Analysis
- PMID: 32064377
- PMCID: PMC7016918
- DOI: 10.1021/acsomega.9b03084
Toxicological Risks of Renqingchangjue in Rats Evaluated by 1H NMR-Based Serum and Urine Metabolomics Analysis
Abstract
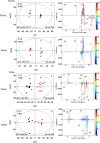
Renqingchangjue (RQCJ), a kind of Traditional Tibetan Medicine, has been widely utilized to treat various gastroenteritis diseases. However, the biosafety and toxicity of RQCJ was still indefinite because of toxic components in RQCJ, which included a variety of heavy metals. Thus, this study was aimed to evaluate the toxicity and expound the toxicological mechanism of RQCJ. In this study, rats were intragastrically administered with different doses of RQCJ for 15 days, and then, the restorative observation period lasted for 15 days. Liver and kidney tissues were collected for histopathological examination, and simultaneously serum and urine samples were collected for 1H nuclear magnetic resonance (1H NMR) spectroscopy analysis and biochemical analysis combined with inductively coupled plasma mass spectrometry (ICP-MS) measurement. The 1H NMR-based metabolomics analysis revealed that the administration of RQCJ significantly altered the concentrations of 14 serum metabolites and 14 urine metabolites, which implied disturbances in energy metabolism, amino acid metabolism, intestinal flora environment, and membrane damage. Besides, the biochemical analysis of serum samples was consistent with the histopathological results, which indicated slight hepatotoxicity and nephrotoxicity. The quantification of As and Hg in urine and serum samples by ICP-MS provided more evidence about the toxicity of RQCJ. This work provided an effective method to systematically and dynamically evaluate the toxicity of RQCJ and suggested that precautions should be taken in the clinic to monitor the potential toxicity of RQCJ.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Liu M.; He Y.; Ge S.; Cheng M.; Xie H.; Yu C.; Wang X. Chemical Form and Bioaccessibility of Mercury in Traditional Tibetan Medicines. Environ. Sci. Technol. Lett. 2018, 5, 552–557. 10.1021/acs.estlett.8b00371. - DOI
-
- Cesnek M.; Štefánik M.; Miglierini M.; Kmječ T.; Sklenka L. U. Analysis of traditional Tibetan pills. Hyperfine Interact. 2017, 238, 93.10.1007/s10751-017-1469-8. - DOI
-
- Sallon S.; Dory Y.; Barghouthy Y.; Tamdin T.; Sangmo R.; Tashi J.; Yangdon S.; Yeshi T.; Sadutshang T.; Rotenberg M.; Cohen E.; Harlavan Y.; Sharabi G.; Bdolah-Abram T. Is mercury in Tibetan Medicine toxic? Clinical, neurocognitive and biochemical results of an initial cross-sectional study. Exp. Biol. Med. 2017, 242, 316–332. 10.1177/1535370216672748. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
