Exploring Possible Surrogates for Kobayashi's Aryne Precursors
- PMID: 32064404
- PMCID: PMC7017410
- DOI: 10.1021/acsomega.9b03989
Exploring Possible Surrogates for Kobayashi's Aryne Precursors
Abstract
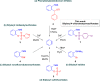
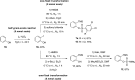
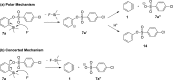
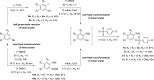
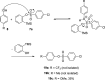
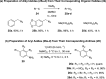
A class of aryne precursors, that is, 2-(trimethylsilyl)aryl 4-chlorobenzenesulfonates, has been developed through well-established synthetic routes, which allow the formation of arynes under relatively mild conditions. All the aryne precursors were obtained from phenols and 4-chlorobenzenesulfonyl chloride, an inexpensive and easy-to-handle reagent with relatively low toxicity, and subjected to nucleophilic addition reactions, providing addition products in yields of 24 to 92%, and to cycloaddition reactions, affording cycloadducts in yields up to 80%. This work provides interesting insights into the mechanisms of aryne generation. In addition, 2-(trimethylsilyl)phenyl 4-chlorobenzenesulfonate was successfully employed in the total synthesis of (±)-aporphine.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
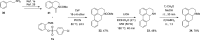
Similar articles
-
Use of benzynes for the synthesis of heterocycles.Org Biomol Chem. 2013 Jan 14;11(2):191-218. doi: 10.1039/c2ob26673c. Epub 2012 Nov 6. Org Biomol Chem. 2013. PMID: 23132413
-
Access to diverse organosulfur compounds via arynes: a comprehensive review on Kobayashi's aryne precursor.Org Biomol Chem. 2021 Oct 14;19(39):8466-8481. doi: 10.1039/d1ob01436f. Org Biomol Chem. 2021. PMID: 34568887 Review.
-
Exploration of Kobayashi's aryne precursor: a potent reactive platform for the synthesis of polycyclic aromatic hydrocarbons.Org Biomol Chem. 2021 Jan 28;19(4):722-737. doi: 10.1039/d0ob02063j. Epub 2021 Jan 12. Org Biomol Chem. 2021. PMID: 33432965
-
Employing Arynes in Diels-Alder Reactions and Transition-Metal-Free Multicomponent Coupling and Arylation Reactions.Acc Chem Res. 2016 Sep 20;49(9):1658-70. doi: 10.1021/acs.accounts.6b00188. Epub 2016 Aug 25. Acc Chem Res. 2016. PMID: 27560296
-
Aryne Chemistry: Generation Methods and Reactions Incorporating Multiple Arynes.Chem Rev. 2024 Oct 23;124(20):11435-11522. doi: 10.1021/acs.chemrev.4c00296. Epub 2024 Oct 9. Chem Rev. 2024. PMID: 39383091 Review.
References
-
-
For reviews, see:
- Takikawa H.; Nishii A.; Sakai T.; Suzuki K. Aryne-Based Strategy in the Total Synthesis of Naturally Occuring Polycyclic Compounds. Chem. Soc. Rev. 2018, 47, 8030–8056. 10.1039/C8CS00350E. - DOI - PubMed
- Tadross P. M.; Stoltz B. M. A Comprehensive History of Arynes in Natural Product Total Synthesis. Chem. Rev. 2012, 112, 3550–3577. 10.1021/cr200478h. - DOI - PubMed
- Gampe C. M.; Carreira E. M. Arynes and Cyclohexyne in Natural Product Synthesis. Angew. Chem., Int. Ed. 2012, 51, 3766–3778. 10.1002/anie.201107485. - DOI - PubMed
-
For selected examples, see:
- Corsello M. A.; Kim J.; Garg N. K. Total Synthesis of (−)-Tubingensin B Enabled by the Strategic Use of an Aryne Cyclization. Nat. Chem. 2017, 9, 944–949. 10.1038/nchem.2801. - DOI - PMC - PubMed
- Muraca A. C. A.; Perecim G. P.; Rodrigues A.; Raminelli C. Convergent Total Synthesis of (±)-Apomorphine via Benzyne Chemistry: Insights into the Mechanisms Involved in the Key Step. Synthesis 2017, 49, 3546–3557. 10.1055/s-0036-1588855. - DOI
- Gouthami P.; Chegondi R.; Chandrasekhar S. Formal Total Synthesis of (±)-Cephalotaxine and Congeners via Aryne Insertion Reaction. Org. Lett. 2016, 18, 2044–2046. 10.1021/acs.orglett.6b00659. - DOI - PubMed
- Rossini A. F. C.; Muraca A. C. A.; Casagrande G. A.; Raminelli C. Total Syntheses of Aporphine Alkaloids via Benzyne Chemistry: An Approach to the Formation of Aporphine Cores. J. Org. Chem. 2015, 80, 10033–10040. 10.1021/acs.joc.5b01634. - DOI - PubMed
- Allan K. M.; Stoltz B. M. A Concise Total Synthesis of (−)-Quinocarcin via Aryne Annulation. J. Am. Chem. Soc. 2008, 130, 17270–17271. 10.1021/ja808112y. - DOI - PubMed
-
-
-
For reviews, see:
- Pozo I.; Guitián E.; Pérez D.; Peña D. Synthesis of Nanographenes, Starphenes, and Sterically Congested Polyarenes by Aryne Cyclotrimerization. Acc. Chem. Res. 2019, 52, 2472–2481. 10.1021/acs.accounts.9b00269. - DOI - PubMed
- Roy T.; Biju A. T. Recent Advances in Molecular Rearrangements Involving Aryne Intermediates. Chem. Commun. 2018, 54, 2580–2594. 10.1039/C7CC09122B. - DOI - PubMed
- Shi J.; Li Y.; Li Y. Aryne Multifunctionalization with Benzdiyne and Benztriyne Equivalents. Chem. Soc. Rev. 2017, 46, 1707–1719. 10.1039/C6CS00694A. - DOI - PubMed
- García-López J.-A.; Greaney M. F. Synthesis of Biaryls Using Aryne Intermediates. Chem. Soc. Rev. 2016, 45, 6766–6798. 10.1039/C6CS00220J. - DOI - PubMed
- Wu D.; Ge H.; Liu S. H.; Yin J. Arynes in the Synthesis of Polycyclic Aromatic Hydrocarbons. RSC Adv. 2013, 3, 22727–22738. 10.1039/c3ra43804j. - DOI
- Pérez D.; Peña D.; Guitián E. Aryne Cycloaddition Reactions in the Synthesis of Large Polycyclic Aromatic Compounds. Eur. J. Org. Chem. 2013, 5981–6013. 10.1002/ejoc.201300470. - DOI
-
For selected examples, see:
- Mizukoshi Y.; Mikami K.; Uchiyama M. Aryne Polymerization Enabling Straightforward Synthesis of Elusive Poly(ortho-arylene)s. J. Am. Chem. Soc. 2015, 137, 74–77. 10.1021/ja5112207. - DOI - PubMed
- Li J.; Zhao Y.; Lu J.; Li G.; Zhang J.; Zhao Y.; Sun X.; Zhang Q. Double [4 + 2] Cycloaddition Reaction To Approach a Large Acene with Even-Number Linearly Fused Benzene Rings: 6,9,16,19-Tetraphenyl-1.20,4.5,10.11,14.15-Tetrabenzooctatwista-cene. J. Org. Chem. 2015, 80, 109–113. 10.1021/jo5021163. - DOI - PubMed
- Shen Y.-M.; Grampp G.; Leesakul N.; Hu H.-W.; Xu J.-H. Synthesis and Emitting Properties of the Blue-Light Fluorophores Indolizino[3,4,5-ab]isoindole Derivatives. Eur. J. Org. Chem. 2007, 3718–3726. 10.1002/ejoc.200700250. - DOI
- Chen Y.-L.; Wong M.-S.; Wong W.-Y.; Lee A. W. M. Synthesis and Characterization of Oxadisilole Fused Benzo[b]triphenylene. Tetrahedron Lett. 2007, 48, 2421–2425. 10.1016/j.tetlet.2007.01.174. - DOI
-
-
-
For reviews, see:
- Yoshimura A.; Saito A.; Zhdankin V. V. Iodonium Salts as Benzyne Precursors. Chem. – Eur. J. 2018, 24, 15156–15166. 10.1002/chem.201802111. - DOI - PubMed
- Yoshida S.; Hosoya T. The Renaissance and Bright Future of Synthetic Aryne Chemistry. Chem. Lett. 2015, 44, 1450–1460. 10.1246/cl.150839. - DOI
- Bhunia A.; Yetra S. R.; Biju A. T. Recent Advances in Transition-Metal-Free Carbon–Carbon and Carbon–Heteroatom Bond-Forming Reactions Using Arynes. Chem. Soc. Rev. 2012, 41, 3140–3152. 10.1039/c2cs15310f. - DOI - PubMed
- Kitamura T. Synthetic Methods for the Generation and Preparative Application of Benzyne. Aust. J. Chem. 2010, 63, 987–1001. 10.1071/CH10072. - DOI
- Dyke A. M.; Hester A. J.; Lloyd-Jones G. C. Organometallic Generation and Capture of ortho-Arynes. Synthesis 2006, 4093–4112. 10.1055/s-2006-950370. - DOI
- Pellissier H.; Santelli M. The Use of Arynes in Organic Synthesis. Tetrahedron 2003, 59, 701–730. 10.1016/S0040-4020(02)01563-6. - DOI
-
For selected examples, see:
- Matsumoto T.; Hosoya T.; Katsuki M.; Suzuki K. New Efficient Protocol for Aryne Generation. Selective Synthesis of Differentially Protected 1,4,5-Naphthalenetriols. Tetrahedron Lett. 1991, 32, 6735–6736. 10.1016/S0040-4039(00)93589-5. - DOI
- Campbell C. D.; Rees C. W. Reactive Intermediates. Part II. Some Addition Reactions of Benzyne. J. Chem. Soc. C. 1969, 748–751. 10.1039/j39690000748. - DOI
- Stiles M.; Miller R. G.; Burckhardt U. Reactions of Benzyne Intermediates in Non-basic Media. J. Am. Chem. Soc. 1963, 85, 1792–1797. 10.1021/ja00895a022. - DOI
- Le Goff E. Aprotic Generation of Benzyne from Diphenyliodonium-2-Carboxylate. J. Am. Chem. Soc. 1962, 84, 3786.10.1021/ja00878a050. - DOI
- Wittig G.; Knauss E. Dehydrobenzol und Cyclopentadien. Chem. Ber. 1958, 91, 895–907. 10.1002/cber.19580910502. - DOI
-
-
- Himeshima Y.; Sonoda T.; Kobayashi H. Fluoride-induced 1,2-Elimination of o-Trimethylsilylphenyl Triflate to Benzyne under Mild Conditions. Chem. Lett. 1983, 12, 1211–1214. 10.1246/cl.1983.1211. - DOI
-
-
For reviews, see:
- Dubrovskiy A. V.; Markina N. A.; Larock R. C. Use of Benzynes for the Synthesis of Heterocycles. Org. Biomol. Chem. 2013, 11, 191–218. 10.1039/C2OB26673C. - DOI - PubMed
- Yoshida H.; Takaki K. Aryne Insertion Reactions into Carbon–Carbon σ-Bonds. Synlett 2012, 23, 1725–1732. 10.1055/s-0031-1290401. - DOI
- Yoshida H.; Ohshita J.; Kunai A. Aryne, ortho-Quinone Methide, and ortho-Quinodimethane: Synthesis of Multisubstituted Arenes Using the Aromatic Reactive Intermediates. Bull. Chem. Soc. Jpn. 2010, 83, 199–219. 10.1246/bcsj.20090245. - DOI
-
LinkOut - more resources
Full Text Sources
Miscellaneous

