Activation of autophagy inhibits nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome activation and attenuates myocardial ischemia-reperfusion injury in diabetic rats
- PMID: 32064785
- PMCID: PMC7477534
- DOI: 10.1111/jdi.13235
Activation of autophagy inhibits nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome activation and attenuates myocardial ischemia-reperfusion injury in diabetic rats
Abstract
Aims/introduction: Diabetic hearts are more vulnerable to ischemia-reperfusion injury (I/RI). The activation of nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome can mediate the inflammatory process, and hence might contribute to myocardial I/RI. Activation of autophagy can eliminate excess reactive oxygen species and alleviate myocardial I/RI in diabetes. The present study aimed to investigate whether the activation of autophagy can alleviate diabetic myocardial I/RI through inhibition of NLRP3 inflammasome activation.
Materials and methods: A dose of 65 mg/kg streptozotocin was given by tail vein injection to establish a type 1 diabetes model in the rats. The left anterior descending coronary artery was ligated for 30 min followed by reperfusion for 2 h to establish a myocardial I/RI model. H9C2 cardiomyocytes were exposed to high glucose (33 mmol/L) and subjected to hypoxia-reoxygenation (6 h hypoxia followed by 4 h reoxygenation).
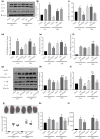
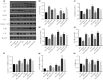
Results: The diabetic rats showed significant inhibition of cardiac autophagy (decreased LC3-II/I and increased p62) that was concomitant with increased activation of NLRP3 inflammasome (increased NLRP3, apoptosis-related spots protein cleaved caspase-1, interleukin-18, interleukin-1β) and more severe myocardial I/RI (elevated creatine kinase myocardial band, lactate dehydrogenase and larger infarct size). However, administration of rapamycin, an inhibitor of the autophagy, to activate autophagy resulted in the inhibition of NLRP3 inflammasome, and finally alleviated myocardial I/RI. In vitro, high glucose inhibited autophagy, while activating NLRP3 inflammasome in H9C2 cardiomyocytes and aggravating hypoxia-reoxygenation injury, but rapamycin reversed these adverse effects of high glucose.
Conclusion: Activation of autophagy can suppress the formation of NLRP3 inflammasome, which in turn attenuates myocardial ischemia-reperfusion injury in diabetic rats.
Keywords: Diabetic myocardium; Myocardial ischemia-reperfusion injury; Nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome.
© 2020 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Lipopolysaccharide (LPS) Aggravates High Glucose- and Hypoxia/Reoxygenation-Induced Injury through Activating ROS-Dependent NLRP3 Inflammasome-Mediated Pyroptosis in H9C2 Cardiomyocytes.J Diabetes Res. 2019 Feb 17;2019:8151836. doi: 10.1155/2019/8151836. eCollection 2019. J Diabetes Res. 2019. PMID: 30911553 Free PMC article.
-
NLRP3 Inflammasome Activation-Mediated Pyroptosis Aggravates Myocardial Ischemia/Reperfusion Injury in Diabetic Rats.Oxid Med Cell Longev. 2017;2017:9743280. doi: 10.1155/2017/9743280. Epub 2017 Sep 14. Oxid Med Cell Longev. 2017. PMID: 29062465 Free PMC article.
-
Cardioprotective effect of cinnamaldehyde pretreatment on ischemia/ reperfusion injury via inhibiting NLRP3 inflammasome activation and gasdermin D mediated cardiomyocyte pyroptosis.Chem Biol Interact. 2022 Dec 1;368:110245. doi: 10.1016/j.cbi.2022.110245. Epub 2022 Oct 29. Chem Biol Interact. 2022. PMID: 36341777
-
Myocardial ischemia-reperfusion injury: The balance mechanism between mitophagy and NLRP3 inflammasome.Life Sci. 2024 Oct 15;355:122998. doi: 10.1016/j.lfs.2024.122998. Epub 2024 Aug 20. Life Sci. 2024. PMID: 39173998 Review.
-
Cell-Specific Roles of NLRP3 Inflammasome in Myocardial Infarction.J Cardiovasc Pharmacol. 2019 Sep;74(3):188-193. doi: 10.1097/FJC.0000000000000709. J Cardiovasc Pharmacol. 2019. PMID: 31356542 Review.
Cited by
-
The mechanism by which miR-494-3p regulates PGC1-α-mediated inhibition of mitophagy in cardiomyocytes and alleviation of myocardial ischemia-reperfusion injury.BMC Cardiovasc Disord. 2023 Apr 21;23(1):204. doi: 10.1186/s12872-023-03226-7. BMC Cardiovasc Disord. 2023. PMID: 37085803 Free PMC article.
-
Melatonin Reduces NLRP3 Inflammasome Activation by Increasing α7 nAChR-Mediated Autophagic Flux.Antioxidants (Basel). 2020 Dec 18;9(12):1299. doi: 10.3390/antiox9121299. Antioxidants (Basel). 2020. PMID: 33353046 Free PMC article.
-
Platelet-rich plasma alleviates skin photoaging by activating autophagy and inhibiting inflammasome formation.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jul;398(7):8669-8680. doi: 10.1007/s00210-025-03800-0. Epub 2025 Jan 21. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39836253
-
Lycopene alleviates hepatic ischemia reperfusion injury via the Nrf2/HO-1 pathway mediated NLRP3 inflammasome inhibition in Kupffer cells.Ann Transl Med. 2021 Apr;9(8):631. doi: 10.21037/atm-20-7084. Ann Transl Med. 2021. PMID: 33987329 Free PMC article.
-
Pyroptosis and Its Regulation in Diabetic Cardiomyopathy.Front Physiol. 2022 Jan 25;12:791848. doi: 10.3389/fphys.2021.791848. eCollection 2021. Front Physiol. 2022. PMID: 35145423 Free PMC article. Review.
References
-
- Rawshani A, Rawshani A, Franzen S, et al Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2018; 379: 633–644. - PubMed
-
- Davidson SM, Ferdinandy P, Andreadou I, et al Multitarget Strategies to Reduce Myocardial Ischemia/Reperfusion Injury: JACC Review Topic of the Week. J Am Coll Cardiol 2019; 73: 89–99. - PubMed
-
- Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol 2018; 15: 203–214. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

