Cost-Effectiveness Model Shows Superiority of Wireless Spinal Cord Stimulation Implantation Without a Separate Trial
- PMID: 32065696
- PMCID: PMC8246551
- DOI: 10.1111/ner.13102
Cost-Effectiveness Model Shows Superiority of Wireless Spinal Cord Stimulation Implantation Without a Separate Trial
Abstract
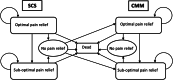
Objective: We evaluated the cost-effectiveness of wireless spinal cord stimulation (Wireless SCS) with single stage "direct to permanent" implantation vs. screening with temporary electrodes and an external pulse generator followed by implantation of a system for long-term use (IPG SCS).
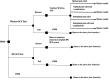
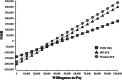
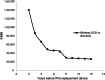
Materials and methods: We created a cost model that takes a 2019 United States (U.S.) payer perspective and is based on IPG SCS cost models for subjects with chronic back and/or leg pain. Our six-month decision tree includes the screening trial period (success ≥50% relief) and leads to various levels of pain relief with or without complications for IPG SCS and Wireless SCS and without complications for conventional medical management (CMM). Every three months in the follow-on 15-year Markov model (with costs and quality-adjusted life years discounted 3.5% annually), subjects remain stable or transition to deteriorated health or death. Subjects who fail SCS receive CMM. After 60 Markov cycles, a 100,000-sample simulation reveals the impact of maximum willingness-to-pay (WTP) from $10,000 to $100,000 per quality-adjusted life year on net monetary benefit (NMB). Sensitivity analyses considered the impact of the Wireless SCS screening success rate, Wireless SCS device cost, and IPG SCS device longevity.
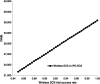
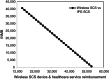
Results: Compared with IPG SCS, Wireless SCS offers higher clinical effectiveness at a lower cost and a higher NMB for our WTP thresholds and is, thus, dominant. Wireless SCS is also cost-effective compared with CMM. Results remain robust with 1) Wireless SCS screening success rates as low as 85% (dominant), 2) the cost of the Wireless SCS devices as high as $55,000 (cost-effective), and 3) IPG SCS devices lasting 12 years (dominant).
Conclusions: In this model, compared with IPG SCS or with CMM, Wireless SCS is a superior strategy.
Keywords: Cost-effectiveness; SCS health economics; modeling study; spinal cord stimulation; wireless SCS.
© 2020 The Authors. Neuromodulation: Technology at the Neural Interface published by Wiley Periodicals, Inc. on behalf of International Neuromodulation Society.
Figures


References
-
- Feler CA, Kaufman S. Spinal cord stimulation: one stage? Acta Neurochir 1992;117:91.
-
- Oakley JC, Krames ES, Stamatos J, Foster AM. Successful long‐term outcomes of spinal cord stimulation despite limited pain relief during temporary trialing. Neuromodulation 2008;11:66–73. - PubMed
-
- Weinand ME, Madhusudan H, Davis B, Melgar M. Acute vs prolonged screening for spinal cord stimulation in chronic pain. Neuromodulation 2003;6:15–19. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

