Stereoselective Synthesis of New (2 S,3 R)-3-Carboxyphenyl)pyrrolidine-2-carboxylic Acid Analogues Utilizing a C(sp3)-H Activation Strategy and Structure-Activity Relationship Studies at the Ionotropic Glutamate Receptors
- PMID: 32065744
- PMCID: PMC7227796
- DOI: 10.1021/acschemneuro.0c00003
Stereoselective Synthesis of New (2 S,3 R)-3-Carboxyphenyl)pyrrolidine-2-carboxylic Acid Analogues Utilizing a C(sp3)-H Activation Strategy and Structure-Activity Relationship Studies at the Ionotropic Glutamate Receptors
Abstract
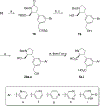
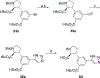
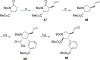
Competitive antagonists for ionotropic glutamate receptors (iGluRs) are highly valuable tool compounds for studying health and disease states in the central nervous system. However, only few subtype selective tool compounds are available and the discovery of antagonists with novel iGluR subtype selectivity profiles remains a profound challenge. In this paper, we report an elaborate structure-activity relationship (SAR) study of the parental scaffold 2,3-trans-3-carboxy-3-phenyl-proline by the synthesis of 40 new analogues. Three synthetic strategies were employed with two new strategies of which one being a highly efficient and fully enantioselective strategy based on C(sp3)-H activation methodology. The SAR study led to the conclusion that selectivity for the NMDA receptors was a general trend when adding substituents in the 5'-position. Selective NMDA receptor antagonists were obtained with high potency (IC50 values as low as 200 nM) and 3-34-fold preference for GluN1/GluN2A over GluN1/GluN2B-D NMDA receptors.
Keywords: C(sp3)−H activation; Electrophysiology; Ionotropic glutamate receptors; NMDA receptor antagonist; Proline analogues.
Figures











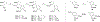
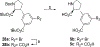

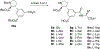

References
-
- Meldrum BS Glutamate as a Neurotransmitter in the Brain: Review of Physiology and Pathology. J. Nutr 2000, 130 (4), 1007S–1015S. - PubMed
-
- Riaza Bermudo-Soriano C; Perez-Rodriguez MM; Vaquero-Lorenzo C; Baca-Garcia E New Perspectives in Glutamate and Anxiety. Pharmacol. Biochem. Behav 2012, 100 (4), 752–774. - PubMed
-
- McCarthy DJ; Alexander R; Smith MA; Pathak S; Kanes S; Lee CM; Sanacora G Glutamate-Based Depression GBD. Med. Hypotheses 2012, 78 (5), 675–681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

