Asymmetric Synthesis of [2.2.2]-Bicyclic Lactones via All-Carbon Inverse-Electron-Demand Diels-Alder Reaction
- PMID: 32065757
- PMCID: PMC7497662
- DOI: 10.1021/acs.orglett.0c00138
Asymmetric Synthesis of [2.2.2]-Bicyclic Lactones via All-Carbon Inverse-Electron-Demand Diels-Alder Reaction
Abstract
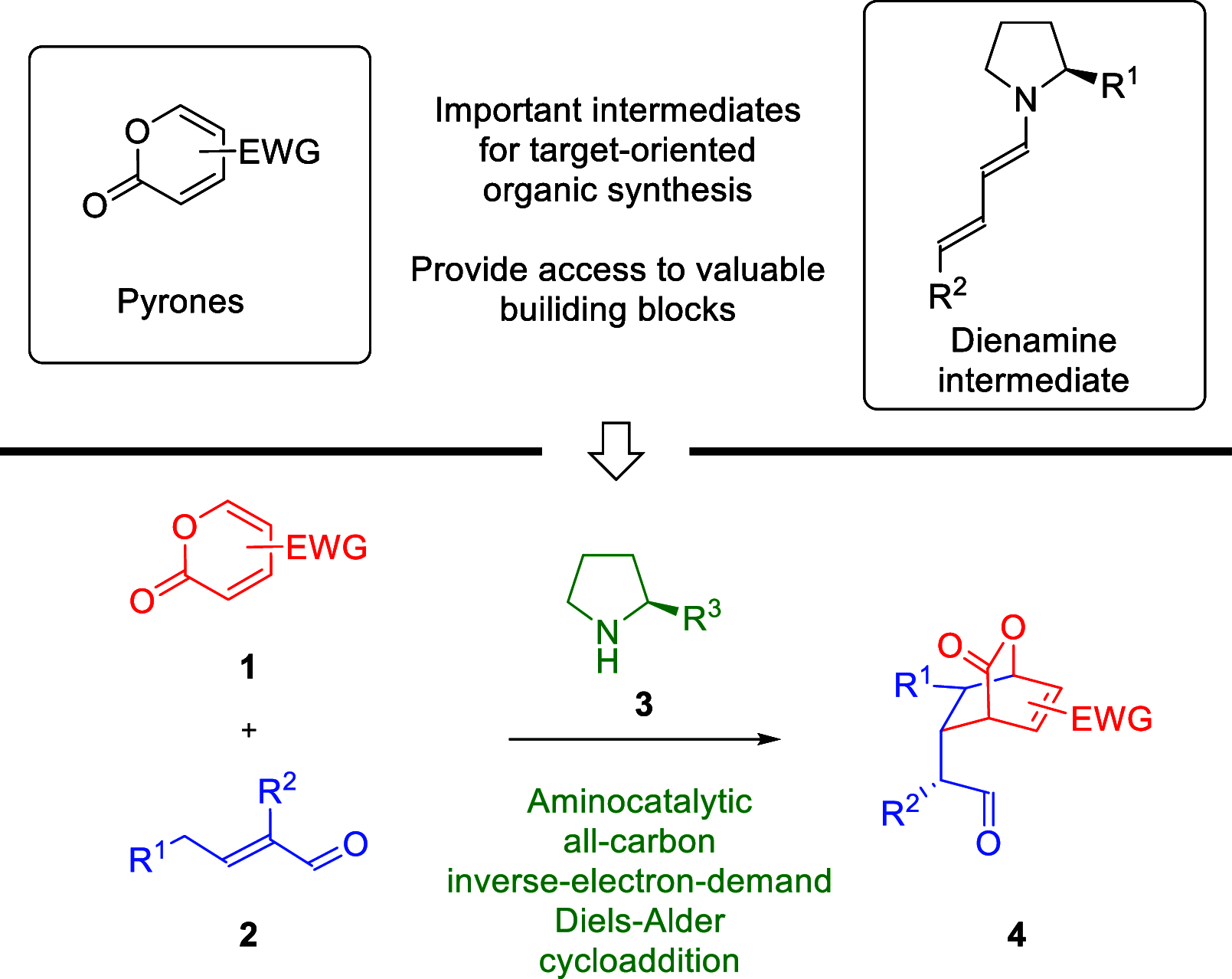
In this paper, a new cycloaddition between α,β-unsaturated aldehydes and coumalates realized under dienamine activation has been described. The reaction proceeds regioselectively with the distal double bond of the dienamine system acting as electron-rich dienophile. It leads to the formation of biologically relevant [2.2.2]-bicyclic lactones. Their functionalization potential has been confirmed in selected, diastereoselective transformations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Aminocatalytic asymmetric Diels-Alder reactions via HOMO activation.Acc Chem Res. 2012 Sep 18;45(9):1491-500. doi: 10.1021/ar3000822. Epub 2012 Jun 20. Acc Chem Res. 2012. PMID: 22716926
-
Asymmetric pyrone Diels-Alder reactions enabled by dienamine catalysis.Chem Sci. 2019 Dec 26;11(8):2175-2180. doi: 10.1039/c9sc05738b. Chem Sci. 2019. PMID: 34123308 Free PMC article.
-
Inverse-electron-demand Diels-Alder reactions of α,β-unsaturated hydrazones with 3-methoxycarbonyl α-pyrones.Org Biomol Chem. 2018 Nov 28;16(46):8913-8916. doi: 10.1039/c8ob02132e. Org Biomol Chem. 2018. PMID: 30422144
-
Catalytic Asymmetric Inverse-Electron-Demand Hetero-Diels-Alder Reactions.Chem Rec. 2017 Dec;17(12):1184-1202. doi: 10.1002/tcr.201700006. Epub 2017 May 16. Chem Rec. 2017. PMID: 28508470 Review.
-
Inverse-Electron-Demand Diels-Alder Reactions of 2-Pyrones: Bridged Lactones and Beyond.Chemistry. 2021 Mar 12;27(15):4760-4788. doi: 10.1002/chem.202003980. Epub 2021 Jan 12. Chemistry. 2021. PMID: 33085124 Review.
Cited by
-
Oxyenamides as Versatile Building Blocks for a Highly Stereoselective One-Pot Synthesis of the 1,3-Diamino-2-ol-Scaffold Containing Three Continuous Stereocenters.Angew Chem Int Ed Engl. 2021 Oct 25;60(44):23667-23671. doi: 10.1002/anie.202109752. Epub 2021 Sep 28. Angew Chem Int Ed Engl. 2021. PMID: 34463410 Free PMC article.
-
Organocatalytic Asymmetric Approach to γ,δ-Functionalization of 3-Cyano-4-styrylcoumarins via Bifunctional Catalysis.Org Lett. 2022 Oct 28;24(42):7722-7726. doi: 10.1021/acs.orglett.2c02836. Epub 2022 Oct 17. Org Lett. 2022. PMID: 36252955 Free PMC article.
-
tert-Butyl(2-oxo-2H-pyran-5-yl)carbamate as the First Chameleon Diene Bearing an Electron-Donating Substituent.Molecules. 2022 Sep 2;27(17):5666. doi: 10.3390/molecules27175666. Molecules. 2022. PMID: 36080432 Free PMC article.
-
Iridium-catalyzed enantioselective olefinic C(sp2)-H allylic alkylation.Chem Sci. 2021 Jan 15;12(8):3070-3075. doi: 10.1039/d0sc06208a. Chem Sci. 2021. PMID: 34164076 Free PMC article.
References
-
- Büchi G.; Erickson R. E.; Wakabayashi N. J. Am. Chem. Soc. 1961, 83, 927.10.1021/ja01465a042. - DOI
- Wolff G.; Ourisson G. Tetrahedron 1969, 25, 4903.10.1016/S0040-4020(01)83031-3. - DOI
- Terhune S. J.; Hogg J. W.; Lawrence B. M. Tetrahedron Lett. 1973, 14, 4705.10.1016/S0040-4039(01)87315-9. - DOI
- Dunn A. W.; Johnstone R. A. W.; Sklarz B.; King T. J. J. Chem. Soc., Chem. Commun. 1976, 270a.10.1039/c3976000270a. - DOI
- Dunn A. W.; Johnstone R. A. W.; Sklarz B.; Lessinger L.; King T. J. J. Chem. Soc., Chem. Commun. 1978, 533.10.1039/c39780000533. - DOI
- Kobayashi J.; Ueno S.; Morita H. J. Org. Chem. 2002, 67, 6546.10.1021/jo0258204. - DOI - PubMed
- Morita H.; Ishioka N.; Takatsu H.; Iizuka T.; Kobayashi J. J. Nat. Prod. 2006, 69, 418.10.1021/np0503799. - DOI - PubMed
- Hao X.; Shen Y.; Li L.; He H. Curr. Med. Chem. 2003, 10, 2253.10.2174/0929867033456684. - DOI - PubMed
- Li S.-H.; Wang J.; Niu X.-M.; Shen J.-H.; Zhang H.-J.; Sun H.-D.; Li M.-L.; Tian Q.-E.; Lu Y.; Cao P.; Zheng Q.-T. Org. Lett. 2004, 6, 4327.10.1021/ol0481535. - DOI - PubMed
- Rakotonandrasana O. L.; Raharinjato F. H.; Rajaonarivelo M.; Dumontet V.; Martin M.-T.; Bignon J.; Rasoanaivo P. J. Nat. Prod. 2010, 73, 1730.10.1021/np1005086. - DOI - PubMed
- Moustafa G. A. I.; Nojima S.; Yamano Y.; Aono A.; Arai M.; Mitarai S.; Tanaka T.; Yoshimitsu T. MedChemComm 2013, 4, 720.10.1039/c3md00016h. - DOI
-
- Cai X.-H.; Tan Q.-G.; Liu Y.-P.; Feng T.; Du Z.-Z.; Li W.-Q.; Luo X.-D. Org. Lett. 2008, 10, 577.10.1021/ol702682h. - DOI - PubMed
- Asakawa Y.; Toyota M.; Taira Z.; Takemoto T.; Kido M.; Ichikawa Y. J. Chem. Soc., Chem. Commun. 1980, 1232.10.1039/C39800001232. - DOI
- Nakashima K.; Kawano H.; Kumano M.; Kodama H.; Kameoka M.; Yamamoto A.; Mizutani R.; Sono M.; Tori M. Tetrahedron Lett. 2015, 56, 4912.10.1016/j.tetlet.2015.06.083. - DOI
- Du J.; Chiu M.-H.; Nie R.-L. J. Nat. Prod. 1999, 62, 1664.10.1021/np9900270. - DOI
- Buta J. G.; Flippen J. L.; Lusby W. R. J. Org. Chem. 1978, 43, 1002.10.1021/jo00399a047. - DOI
- Evanno L.; Jossang A.; Nguyen-Pouplin J.; Delaroche D.; Herson P.; Seuleiman M.; Bodo B.; Nay B. Planta Med. 2008, 74, 870.10.1055/s-2008-1074546. - DOI - PubMed
- Xiong L.; Zhou Q.-M.; Zou Y.; Chen M.-H.; Guo L.; Hu G.-Y.; Liu Z.-H.; Peng C. Org. Lett. 2015, 17, 6238.10.1021/acs.orglett.5b03227. - DOI - PubMed
-
- Konkel M. J.; Vince V. Tetrahedron 1996, 52, 799.10.1016/0040-4020(95)00926-4. - DOI
- Trotter N. S.; Larsen D. S.; Stoodley R. J.; Brooker S. Tetrahedron Lett. 2000, 41, 8957.10.1016/S0040-4039(00)01589-6. - DOI
- Nicolaou K. C.; Snyder S. A.; Montagnon T.; Vassilikogiannakis G. Angew. Chem., Int. Ed. 2002, 41, 1668.10.1002/1521-3773(20020517)41:10<1668::AID-ANIE1668>3.0.CO;2-Z. - DOI - PubMed
- Larsen D. S.; Lins R. J.; Stoodley R. J.; Trotter N. S. Org. Biomol. Chem. 2004, 2, 1934.10.1039/b404688a. - DOI - PubMed
- Abdelkafi H.; Herson P.; Nay B. Org. Lett. 2012, 14, 1270.10.1021/ol300133x. - DOI - PubMed
- Grant P. S.; Brimble M. A.; Furkert D. P. Chem. - Asian J. 2019, 14, 1128.10.1002/asia.201800903. - DOI - PubMed
-
- Posner G. H.; Carry J. K.; Lee J. K.; Bull D. S.; Dai S. Tetrahedron Lett. 1994, 35, 1321.10.1016/S0040-4039(00)76207-1. - DOI
- Wang Y.; Li H.; Wang Y.-Q.; Liu Y.; Foxman B. M.; Deng L. J. Am. Chem. Soc. 2007, 129, 6364.10.1021/ja070859h. - DOI - PMC - PubMed
- Singh R. P.; Bartelson K.; Wang Y.; Su H.; Lu X.; Deng L. J. Am. Chem. Soc. 2008, 130, 2422.10.1021/ja078251w. - DOI - PubMed
- Zheng S.; Lu X. Org. Lett. 2009, 11, 3978.10.1021/ol901618h. - DOI - PubMed
- Gutekunst W. R.; Baran P. S. J. Am. Chem. Soc. 2011, 133, 19076.10.1021/ja209205x. - DOI - PMC - PubMed
- Gutekunst W. R.; Gianatassio R.; Baran P. S. Angew. Chem. 2012, 124, 7625.10.1002/ange.201203897. - DOI - PMC - PubMed
- She N.; Zhuo L.; Jiang W.; Zhu X.; Wang J.; Ming Z.; Zhao X.; Cong X.; Huang W. Bioorg. Med. Chem. Lett. 2014, 24, 3351.10.1016/j.bmcl.2014.05.097. - DOI - PubMed
- Liu K.; Teng H.-L.; Wang C.-J. Org. Lett. 2014, 16, 4508.10.1021/ol5020569. - DOI - PubMed
- Gordon J. R.; Nelson H. M.; Virgil S. C.; Stoltz B. M. J. Org. Chem. 2014, 79, 9740.10.1021/jo501924u. - DOI - PubMed
- Shi L.-M.; Dong W.-W.; Tao H.-Y.; Dong X.-Q.; Wang C.-J. Org. Lett. 2017, 19, 4532.10.1021/acs.orglett.7b02107. - DOI - PubMed
- Zhou Y.; Zhou Z.; Du W.; Chen Y.-C. Huaxue Xuebao 2018, 76, 382.10.6023/A18040131. - DOI
- Liu Q.; Zu L. Angew. Chem., Int. Ed. 2018, 57, 9505.10.1002/anie.201805019. - DOI - PubMed
- Pfennig T.; Chemburkar A.; Johnson R. L.; Ryan M. J.; Rossini A. J.; Neurock M.; Shanks B. H. ACS Catal. 2018, 8, 2450.10.1021/acscatal.7b04311. - DOI
- Giardinetti M.; Jessen N. I.; Christensen M. L.; Jørgensen K. A. Chem. Commun. 2019, 55, 202.10.1039/C8CC08551J. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

