Copper-mediated synthesis of drug-like bicyclopentanes
- PMID: 32066140
- PMCID: PMC7148169
- DOI: 10.1038/s41586-020-2060-z
Copper-mediated synthesis of drug-like bicyclopentanes
Abstract
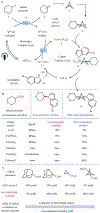
Multicomponent reactions are relied on in both academic and industrial synthetic organic chemistry owing to their step- and atom-economy advantages over traditional synthetic sequences1. Recently, bicyclo[1.1.1]pentane (BCP) motifs have become valuable as pharmaceutical bioisosteres of benzene rings, and in particular 1,3-disubstituted BCP moieties have become widely adopted in medicinal chemistry as para-phenyl ring replacements2. These structures are often generated from [1.1.1]propellane via opening of the internal C-C bond through the addition of either radicals or metal-based nucleophiles3-13. The resulting propellane-addition adducts are then transformed to the requisite polysubstituted BCP compounds via a range of synthetic sequences that traditionally involve multiple chemical steps. Although this approach has been effective so far, a multicomponent reaction that enables single-step access to complex and diverse polysubstituted drug-like BCP products would be more time efficient compared to current stepwise approaches. Here we report a one-step three-component radical coupling of [1.1.1]propellane to afford diverse functionalized bicyclopentanes using various radical precursors and heteroatom nucleophiles via a metallaphotoredox catalysis protocol. This copper-mediated reaction operates on short timescales (five minutes to one hour) across multiple (more than ten) nucleophile classes and can accommodate a diverse array of radical precursors, including those that generate alkyl, α-acyl, trifluoromethyl and sulfonyl radicals. This method has been used to rapidly prepare BCP analogues of known pharmaceuticals, one of which is substantially more metabolically stable than its commercial progenitor.
Conflict of interest statement
Figures




References
-
- Mykhailiuk PK Saturated bioisosteres of benzene: where to go next? Org. Biomol. Chem 17, 2839–2849 (2019). - PubMed
-
- Kanazawa J & Uchiyama M Recent advances in the synthetic chemistry of bicyclo[1.1.1]pentane. Synlett 30, 1–11 (2019).
-
- Kanazawa J, Maeda K & Uchiyama M Radical multicomponent carboamination of [1.1.1]propellane. J. Am. Chem. Soc. 139, 17791–17794 (2017). - PubMed
-
- Nugent J, Arroniz C, Shire BR, Sterling AJ, Pickford HD, Wong MLJ, Mansfield SJ, Caputo DFJ, Owen B, Mousseau JJ, Duarte F & Anderson EA A general route to bicyclo[1.1.1]pentanes through photoredox catalysis. ACS Catal 9, 9568–9574 (2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

