Treatment of rats with spinal cord injury using human bone marrow-derived stromal cells prepared by negative selection
- PMID: 32066435
- PMCID: PMC7026953
- DOI: 10.1186/s12929-020-00629-y
Treatment of rats with spinal cord injury using human bone marrow-derived stromal cells prepared by negative selection
Abstract
Background: Spinal cord injury (SCI) is a highly debilitating pathology without curative treatment. One of the most promising disease modifying strategies consists in the implantation of stem cells to reduce inflammation and promote neural regeneration. In the present study we tested a new human bone marrow-derived stromal cell preparation (bmSC) as a therapy of SCI.
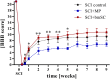
Methods: Spinal cord contusion injury was induced in adult male rats at thoracic level T9/T10 using the Infinite Horizon impactor. One hour after lesion the animals were treated with a sub-occipital injection of human bmSC into the cisterna magna. No immune suppression was used. One dose of bmSC consisted, on average, of 2.3 million non-manipulated cells in 100 μL suspension, which was processed out of fresh human bone marrow from the iliac crest of healthy volunteers. Treatment efficacy was compared with intraperitoneal injections of methylprednisolone (MP) and saline. The recovery of motor functions was assessed during a surveillance period of nine weeks. Adverse events as well as general health, weight and urodynamic functions were monitored daily. After this time, the animals were perfused, and the spinal cord tissue was investigated histologically.
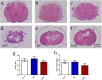
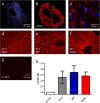
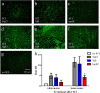
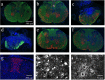
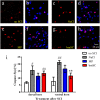
Results: Rats treated with bmSC did not reject the human implants and showed no sign of sickness behavior or neuropathic pain. Compared to MP treatment, animals displayed better recovery of their SCI-induced motor deficits. There were no significant differences in the recovery of bladder control between groups. Histological analysis at ten weeks after SCI revealed no differences in tissue sparing and astrogliosis, however, bmSC treatment was accompanied with reduced axonal degeneration in the dorsal ascending fiber tracts, lower Iba1-immunoreactivity (IR) close to the lesion site and reduced apoptosis in the ventral grey matter. Neuroinflammation, as evidenced by CD68-IR, was significantly reduced in the MP-treated group.
Conclusions: Human bmSC that were prepared by negative selection without expansion in culture have neuroprotective properties after SCI. Given the effect size on motor function, implantation in the acute phase was not sufficient to induce spinal cord repair. Due to their immune modulatory properties, allogeneic implants of bmSC can be used in combinatorial therapies of SCI.
Keywords: Bone marrow; Human; Inflammation; Rat; Spinal cord injury; Stem cells.
Conflict of interest statement
JdM and EChW are CEOs and owners of Neuroplast BV.
Figures




References
-
- Kang Y, Ding H, Zhou H, Wei Z, Liu L, Pan D, Feng S. Epidemiology of worldwide spinal cord injury: a literature review. J Neuro-Oncol. 2018;6:1–9.
-
- Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings MG, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PLJ, Piepmeier J, Sonntag VK, Wagner F, Winn HR, Young W. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results Third Natl Acute Spinal Cord Inj Randomized Control Trial J Neurosurg. 1998;89:699–706. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

