Antiviral treatment perspective against Borna disease virus 1 infection in major depression: a double-blind placebo-controlled randomized clinical trial
- PMID: 32066504
- PMCID: PMC7027224
- DOI: 10.1186/s40360-020-0391-x
Antiviral treatment perspective against Borna disease virus 1 infection in major depression: a double-blind placebo-controlled randomized clinical trial
Abstract
Background: Whether Borna disease virus (BDV-1) is a human pathogen remained controversial until recent encephalitis cases showed BDV-1 infection could even be deadly. This called to mind previous evidence for an infectious contribution of BDV-1 to mental disorders. Pilot open trials suggested that BDV-1 infected depressed patients benefitted from antiviral therapy with a licensed drug (amantadine) which also tested sensitive in vitro. Here, we designed a double-blind placebo-controlled randomized clinical trial (RCT) which cross-linked depression and BDV-1 infection, addressing both the antidepressant and antiviral efficacy of amantadine.
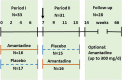
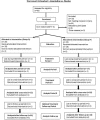
Methods: The interventional phase II RCT (two 7-weeks-treatment periods and a 12-months follow-up) at the Hannover Medical School (MHH), Germany, assigned currently depressed BDV-1 infected patients with either major depression (MD; N = 23) or bipolar disorder (BD; N = 13) to amantadine sulphate (PK-Merz®; twice 100 mg orally daily) or placebo treatment, and contrariwise, respectively. Clinical changes were assessed every 2-3 weeks by the 21-item Hamilton rating scale for depression (HAMD) (total, single, and combined scores). BDV-1 activity was determined accordingly in blood plasma by enzyme immune assays for antigens (PAG), antibodies (AB) and circulating immune complexes (CIC).
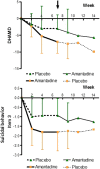
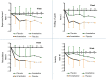
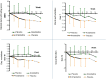
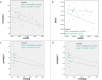
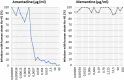
Results: Primary outcomes (≥25% HAMD reduction, week 7) were 81.3% amantadine vs. 35.3% placebo responder (p = 0.003), a large clinical effect size (ES; Cohen's d) of 1.046, and excellent drug tolerance. Amantadine was safe reducing suicidal behaviour in the first 2 weeks. Pre-treatment maximum infection levels were predictive of clinical improvement (AB, p = 0.001; PAG, p = 0.026; HAMD week 7). Respective PAG and CIC levels correlated with AB reduction (p = 0,001 and p = 0.034, respectively). Follow-up benefits (12 months) correlated with dropped cumulative infection measures over time (p < 0.001). In vitro, amantadine concentrations as low as 2.4-10 ng/mL (50% infection-inhibitory dose) prevented infection with human BDV Hu-H1, while closely related memantine failed up to 100,000-fold higher concentration (200 μg/mL).
Conclusions: Our findings indicate profound antidepressant efficacy of safe oral amantadine treatment, paralleling antiviral effects at various infection levels. This not only supports the paradigm of a link of BDV-1 infection and depression. It provides a novel possibly practice-changing low cost mental health care perspective for depressed BDV-1-infected patients addressing global needs.
Trial registration: The trial was retrospectively registered in the German Clinical Trials Registry on 04th of March 2015. The trial ID is DRKS00007649; https://www.drks.de/drks_web/setLocale_EN.do.
Keywords: Borna disease virus 1 (BDV-1); Major depression; amantadine; antiviral treatment; bipolar disorder; double-blind placebo-controlled randomized clinical trial (RCT).
Conflict of interest statement
The authors of this manuscript have the following competing interests: DED and HL held non-licensed common patents on a 2nd indication of amantadine (Germany, USA, China, and Japan) which are expired. HL held non-licensed patents on BDV diagnostic procedures (Germany and USA) which are also expired. HL received personal consulting fees from medical laboratories in Germany, Diamedis (
Figures

Similar articles
-
Amantadine in depressive patients with Borna disease virus (BDV) infection: an open trial.Bipolar Disord. 2000 Mar;2(1):65-70. doi: 10.1034/j.1399-5618.2000.020110.x. Bipolar Disord. 2000. PMID: 11254023 Clinical Trial.
-
Lack of antiviral effect of amantadine in Borna disease virus infection.Med Microbiol Immunol. 1998 Mar;186(4):195-200. doi: 10.1007/s004300050064. Med Microbiol Immunol. 1998. PMID: 9574902
-
Human Borna disease virus-infection and its therapy in affective disorders.APMIS Suppl. 2008;(124):61-5. doi: 10.1111/j.1600-0463.2008.00m10.x. APMIS Suppl. 2008. PMID: 18771101 Review.
-
Borna disease virus is not sensitive to amantadine.Arch Virol. 1997;142(10):2043-8. doi: 10.1007/s007050050221. Arch Virol. 1997. PMID: 9413512
-
Borna disease virus--does it infect humans and cause psychiatric disorders?J Clin Virol. 2001 May;21(2):119-27. doi: 10.1016/s1386-6532(01)00152-4. J Clin Virol. 2001. PMID: 11378492 Review.
Cited by
-
Prominent Efficacy of Amantadine against Human Borna Disease Virus Infection In Vitro and In Vivo. Comment on Fink et al. Amantadine Inhibits SARS-CoV-2 In Vitro. Viruses 2021, 13, 539.Viruses. 2022 Feb 28;14(3):494. doi: 10.3390/v14030494. Viruses. 2022. PMID: 35336901 Free PMC article.
-
Is There such a Thing as Post-Viral Depression?: Implications for Precision Medicine.Biomol Ther (Seoul). 2024 Nov 1;32(6):659-684. doi: 10.4062/biomolther.2024.170. Epub 2024 Oct 21. Biomol Ther (Seoul). 2024. PMID: 39428555 Free PMC article. Review.
-
Microbes and Mental Illness: Past, Present, and Future.Healthcare (Basel). 2023 Dec 29;12(1):83. doi: 10.3390/healthcare12010083. Healthcare (Basel). 2023. PMID: 38200989 Free PMC article. Review.
-
Novel Pharmacological Approaches to the Treatment of Depression.Life (Basel). 2022 Jan 28;12(2):196. doi: 10.3390/life12020196. Life (Basel). 2022. PMID: 35207483 Free PMC article. Review.
-
Exploring New Mechanism of Depression from the Effects of Virus on Nerve Cells.Cells. 2023 Jul 3;12(13):1767. doi: 10.3390/cells12131767. Cells. 2023. PMID: 37443801 Free PMC article. Review.
References
-
- Ferrari AJ, Norman RE, Freedman G, Baxter AJ, Pirkis JE, Harris MG, et al. The burden attributable to mental and substance use disorders as risk factors for suicide: findings from the Global Burden of Disease Study 2010. PLoS One. 2014;9(4):e91936. doi: 10.1371/journal.pone.0091936. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

