The EBMT activity survey on hematopoietic-cell transplantation and cellular therapy 2018: CAR-T's come into focus
- PMID: 32066864
- PMCID: PMC7391287
- DOI: 10.1038/s41409-020-0826-4
The EBMT activity survey on hematopoietic-cell transplantation and cellular therapy 2018: CAR-T's come into focus
Abstract
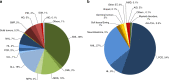
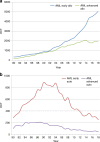
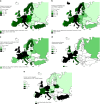
Hematopoietic-cell transplantation (HCT) is widely used for acquired and congenital disorders of the hematopoietic system. Number of transplants performed in Europe and associated countries continues to rise with 47,468 HCT in 42,901 patients [19,630 allogeneic (41%) and 27,838 autologous (59%)] reported by 701 centers in 50 countries in 2018. Main indications were myeloid malignancies 10,679 (25%; 97% allogeneic), lymphoid malignancies 27,318 (64%; 20% allogeneic), solid tumors 1625 (4%; 2.9% allogeneic), and nonmalignant disorders 3063 (7%; 81% allogeneic). This year's analysis focuses on cellular therapies with the marked growth in CAR T-cell therapies from 151 in 2017 to 301 patients reported in 2018. Other cellular therapy numbers show less significant changes. Important trends in HCT include a 49% increase in allogeneic HCT for chronic phase CML (although transplant numbers remain low) and a 24% increase in aplastic anemia. In autologous HCT, there is an ongoing increase in autoimmune diseases (by 19%), predominantly due to activity in multiple sclerosis. This annual report reflects current activity and highlights important trends, useful for health care planning.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Duarte RF, Labopin M, Bader P, Basak GW, Bonini C, Chabannon C, et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant. 2019;54:1525–52. doi: 10.1038/s41409-019-0516-2. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

