Germinal center B cells selectively oxidize fatty acids for energy while conducting minimal glycolysis
- PMID: 32066950
- PMCID: PMC7112716
- DOI: 10.1038/s41590-020-0598-4
Germinal center B cells selectively oxidize fatty acids for energy while conducting minimal glycolysis
Abstract
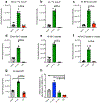
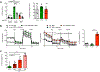
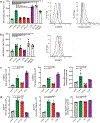
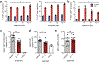
Germinal center B cells (GCBCs) are critical for generating long-lived humoral immunity. How GCBCs meet the energetic challenge of rapid proliferation is poorly understood. Dividing lymphocytes typically rely on aerobic glycolysis over oxidative phosphorylation for energy. Here we report that GCBCs are exceptional among proliferating B and T cells, as they actively oxidize fatty acids (FAs) and conduct minimal glycolysis. In vitro, GCBCs had a very low glycolytic extracellular acidification rate but consumed oxygen in response to FAs. [13C6]-glucose feeding revealed that GCBCs generate significantly less phosphorylated glucose and little lactate. Further, GCBCs did not metabolize glucose into tricarboxylic acid (TCA) cycle intermediates. Conversely, [13C16]-palmitic acid labeling demonstrated that GCBCs generate most of their acetyl-CoA and acetylcarnitine from FAs. FA oxidation was functionally important, as drug-mediated and genetic dampening of FA oxidation resulted in a selective reduction of GCBCs. Hence, GCBCs appear to uncouple rapid proliferation from aerobic glycolysis.
Conflict of interest statement
Competing financial interest
Authors declare that they have no competing financial interest.
Figures












Comment in
-
B is for 'Big Mac': GCs crave a high-fat diet.Nat Immunol. 2020 Mar;21(3):249-250. doi: 10.1038/s41590-020-0614-8. Nat Immunol. 2020. PMID: 32066952 No abstract available.
-
Dietary Fat Makes Germinal Center B Cells Happy.Cell Metab. 2020 May 5;31(5):890-891. doi: 10.1016/j.cmet.2020.04.010. Cell Metab. 2020. PMID: 32375061
-
The fat and the furious: fatty acids fuel hyperproliferative germinal center B cells.Cell Mol Immunol. 2020 Aug;17(8):794-796. doi: 10.1038/s41423-020-0446-3. Epub 2020 May 7. Cell Mol Immunol. 2020. PMID: 32382129 Free PMC article. No abstract available.
References
-
- Shlomchik MJ & Weisel F B cell primary immune responses. Immunol Rev 288, 5–9 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

