Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice
- PMID: 32066981
- PMCID: PMC7065967
- DOI: 10.1038/s41593-020-0589-7
Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice
Abstract
Parkinson's disease is a synucleinopathy that is characterized by motor dysfunction, death of midbrain dopaminergic neurons and accumulation of α-synuclein (α-Syn) aggregates. Evidence suggests that α-Syn aggregation can originate in peripheral tissues and progress to the brain via autonomic fibers. We tested this by inoculating the duodenal wall of mice with α-Syn preformed fibrils. Following inoculation, we observed gastrointestinal deficits and physiological changes to the enteric nervous system. Using the AAV-PHP.S capsid to target the lysosomal enzyme glucocerebrosidase for peripheral gene transfer, we found that α-Syn pathology is reduced due to the increased expression of this protein. Lastly, inoculation of α-Syn fibrils in aged mice, but not younger mice, resulted in progression of α-Syn histopathology to the midbrain and subsequent motor defects. Our results characterize peripheral synucleinopathy in prodromal Parkinson's disease and explore cellular mechanisms for the gut-to-brain progression of α-Syn pathology.
Conflict of interest statement
Figures

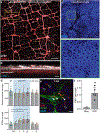



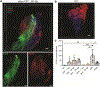
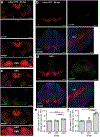






Comment in
-
A route for pathology?Nat Rev Neurosci. 2020 Apr;21(4):179. doi: 10.1038/s41583-020-0288-0. Nat Rev Neurosci. 2020. PMID: 32152522 No abstract available.
References
-
- McCann H, Stevens CH, Cartwright H & Halliday GM α-Synucleinopathy phenotypes. Parkinsonism Relat. Disord 20, S62–S67 (2014). - PubMed
-
- Lees AJ, Hardy J & Revesz T Parkinson’s disease. Lancet 373, 2055–66 (2009). - PubMed
-
- Hawkes CH, Del Tredici K & Braak H A timeline for Parkinson’s disease. Parkinsonism Relat. Disord 16, 79–84 (2010). - PubMed
-
- Sánchez-Ferro Á et al. In vivo gastric detection of α-synuclein inclusions in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc 30, 517–524 (2015). - PubMed
-
- Shannon KM et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc 27, 709–715 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

