STING Pathway Expression Identifies NSCLC With an Immune-Responsive Phenotype
- PMID: 32068166
- PMCID: PMC7202130
- DOI: 10.1016/j.jtho.2020.01.009
STING Pathway Expression Identifies NSCLC With an Immune-Responsive Phenotype
Abstract
Introduction: Although the combination of anti-programmed cell death-1 or anti-programmed cell death ligand-1 (PD-L1) with platinum chemotherapy is a standard of care for NSCLC, clinical responses vary. Even though predictive biomarkers (which include PD-L1 expression, tumor mutational burden, and inflamed immune microenvironment) are validated for immunotherapy, their relevance to chemoimmunotherapy combinations is less clear. We have recently reported that activation of the stimulator of interferon genes (STING) innate immune pathway enhances immunotherapy response in SCLC. Here, we hypothesize that STING pathway activation may predict and underlie predictive correlates of antitumor immunity in NSCLC.
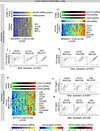
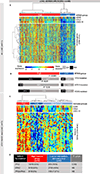
Methods: We analyzed transcriptomic and proteomic profiles in two NSCLC cohorts from our institution (treatment-naive patients in the Profiling of Resistance Patterns and Oncogenic Signaling Pathways in Evaluation of Cancers of the Thorax study and relapsed patients in the Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination study) and The Cancer Genome Atlas (N = 1320). Tumors were stratified by STING activation on the basis of protein or mRNA expression of cyclic GMP-AMP synthase, phospho-STING, and STING-mediated chemokines (chemokine ligand 5 [CCL5] and C-X-C motif chemokine 10 [CXCL10]). STING activation in patient tumors and in platinum-treated preclinical NSCLC models was correlated with biomarkers of immunotherapy response.
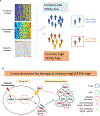
Results: STING activation is associated with higher levels of intrinsic DNA damage, targetable immune checkpoints, and chemokines in treatment-naive and relapsed lung adenocarcinoma. We observed that tumors with lower STING and immune gene expression show higher frequency of serine-threonine kinase 11 (STK11) mutations; however, we identified a subset of these tumors that are TP53 comutated and display high immune- and STING-related gene expression. Treatment with cisplatin increases STING pathway activation and PD-L1 expression in multiple NSCLC preclinical models, including adeno- and squamous cell carcinoma.
Conclusions: STING pathway activation in NSCLC predicts features of immunotherapy response and is enhanced by cisplatin treatment. This suggests a possible predictive biomarker and mechanism for improved response to chemoimmunotherapy combinations.
Keywords: Immune checkpoints; Immunotherapy; Innate immunity; Lung cancer; STING.
Copyright © 2020 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
An Elaborate STING Operation to Take Down NSCLC: Combination of Immunotherapies and Chemotherapies.J Thorac Oncol. 2020 May;15(5):686-688. doi: 10.1016/j.jtho.2020.03.018. J Thorac Oncol. 2020. PMID: 32340674 No abstract available.
References
-
- Ettinger DS, Aisner DL, Wood DE, Akerley W, Bauman J, Chang JY, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw 2018;16:807–21. - PubMed
-
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823–33. - PubMed
-
- Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol 2017;12:403–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

