Antitumor Activity of Asperphenin A, a Lipopeptidyl Benzophenone from Marine-Derived Aspergillus sp. Fungus, by Inhibiting Tubulin Polymerization in Colon Cancer Cells
- PMID: 32069904
- PMCID: PMC7073961
- DOI: 10.3390/md18020110
Antitumor Activity of Asperphenin A, a Lipopeptidyl Benzophenone from Marine-Derived Aspergillus sp. Fungus, by Inhibiting Tubulin Polymerization in Colon Cancer Cells
Abstract
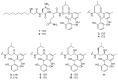
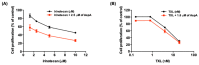
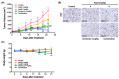
Marine-derived microorganisms are a valuable source of novel bioactive natural products. Asperphenin A is a lipopeptidyl benzophenone metabolite isolated from large-scale cultivation of marine-derived Aspergillus sp. fungus. The compound has shown potent antiproliferative activity against various cancer cells. However, the underlying mechanism of action remained to be elucidated. In this study, we demonstrated the antitumor activity and molecular mechanism of asperphenin A in human colon cancer cells for the first time. Asperphenin A inhibited the growth of colon cancer cells through G2/M cell cycle arrest followed by apoptosis. We further discovered that asperphenin A can trigger microtubule disassembly. In addition to its effect on cell cycle, asperphenin A-induced reactive oxygen species. The compound suppressed the growth of tumors in a colon cancer xenograft model without any overt toxicity and exhibited a combination effect with irinotecan, a topoisomerase I inhibitor. Moreover, we identified the aryl ketone as a key component in the molecular structure responsible for the biological activity of asperphenin A using its synthetic derivatives. Collectively, this study has revealed the antiproliferative and antitumor mechanism of asperphenin A and suggested its possibility as a chemotherapeutic agent and lead compound with a novel structure.
Keywords: Aspergillus sp. fungus; antimitotic agent; asperphenins; colon cancer; marine microorganism metabolite; tubulin polymerization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- National Research Council . From Monsoons to Microbes: Understanding the Ocean’s Role in Human Health. National Academies Press; Washington, DC, USA: 1999. Marine-Derived Pharmaceuticals and Related Bioactive Agents. - PubMed
-
- Li Y., Barbash O., Diehl J.A. 11—Regulation of the Cell Cycle. In: Mendelsohn J., Gray J.W., Howley P.M., Israel M.A., Thompson C.B., editors. The Molecular Basis of Cancer (Fourth Edition) Elsevier Inc.; Philadelphia, PA, USA: 2015. pp. 165–178.e2.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

