Lipophagy-derived fatty acids undergo extracellular efflux via lysosomal exocytosis
- PMID: 32070194
- PMCID: PMC8032247
- DOI: 10.1080/15548627.2020.1728097
Lipophagy-derived fatty acids undergo extracellular efflux via lysosomal exocytosis
Abstract
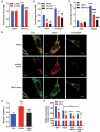
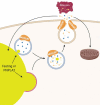
The autophagic degradation of lipid droplets (LDs), termed lipophagy, is a major mechanism that contributes to lipid turnover in numerous cell types. While numerous factors, including nutrient deprivation or overexpression of PNPLA2/ATGL (patatin-like phospholipase domain containing 2) drive lipophagy, the trafficking of fatty acids (FAs) produced from this pathway is largely unknown. Herein, we show that PNPLA2 and nutrient deprivation promoted the extracellular efflux of FAs. Inhibition of autophagy or lysosomal lipid degradation attenuated FA efflux highlighting a critical role for lipophagy in this process. Rather than direct transport of FAs across the lysosomal membrane, lipophagy-derived FA efflux requires lysosomal fusion to the plasma membrane. The lysosomal Ca2+ channel protein MCOLN1/TRPML1 (mucolipin 1) regulates lysosomal-plasma membrane fusion and its overexpression increased, while inhibition blocked FA efflux. In addition, inhibition of autophagy/lipophagy or MCOLN1, or sequestration of extracellular FAs with BSA attenuated the oxidation and re-esterification of lipophagy-derived FAs. Overall, these studies show that the well-established pathway of lysosomal fusion to the plasma membrane is the primary route for the disposal of FAs derived from lipophagy. Moreover, the efflux of FAs and their reuptake or subsequent extracellular trafficking to adjacent cells may play an important role in cell-to-cell lipid exchange and signaling.Abbreviations: ACTB: beta actin; ADRA1A: adrenergic receptor alpha, 1a; ALB: albumin; ATG5: autophagy related 5; ATG7: autophagy related 7; BafA1: bafilomycin A1; BECN1: beclin 1; BHBA: beta-hydroxybutyrate; BSA: bovine serum albumin; CDH1: e-cadherin; CQ: chloroquine; CTSB: cathepsin B; DGAT: diacylglycerol O-acyltransferase; FA: fatty acid; HFD: high-fat diet; LAMP1: lysosomal-associated membrane protein 1; LD: lipid droplet; LIPA/LAL: lysosomal acid lipase A; LLME: Leu-Leu methyl ester hydrobromide; MAP1LC3B/LC3: microtubule associated protein 1 light chain 3 beta; MCOLN1/TRPML1: mucolipin 1; MEF: mouse embryo fibroblast; PBS: phosphate-buffered saline; PIK3C3/VPS34: phosphatidylinositol 3-kinase catalytic subunit type 3; PLIN: perilipin; PNPLA2/ATGL patatin-like phospholipase domain containing 2; RUBCN (rubicon autophagy regulator); SM: sphingomyelin; TAG: triacylglycerol; TMEM192: transmembrane protein 192; VLDL: very low density lipoprotein.
Keywords: Fatty acid; MCOLN1/TRPML1; PNPLA2/ATGL; lipid droplets; lipid metabolism; lipophagy.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures

References
-
- Santambrogio L, Cuervo AM. Chasing the elusive mammalian microautophagy. Autophagy. 2011;7:652–654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
