Thyroid Deficiency Before Birth Alters the Adipose Transcriptome to Promote Overgrowth of White Adipose Tissue and Impair Thermogenic Capacity
- PMID: 32070265
- PMCID: PMC7286741
- DOI: 10.1089/thy.2019.0749
Thyroid Deficiency Before Birth Alters the Adipose Transcriptome to Promote Overgrowth of White Adipose Tissue and Impair Thermogenic Capacity
Abstract
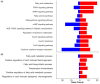
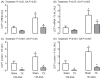
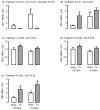
Background: Development of adipose tissue before birth is essential for energy storage and thermoregulation in the neonate and for cardiometabolic health in later life. Thyroid hormones are important regulators of growth and maturation in fetal tissues. Offspring hypothyroid in utero are poorly adapted to regulate body temperature at birth and are at risk of becoming obese and insulin resistant in childhood. The mechanisms by which thyroid hormones regulate the growth and development of adipose tissue in the fetus, however, are unclear. Methods: This study examined the structure, transcriptome, and protein expression of perirenal adipose tissue (PAT) in a fetal sheep model of thyroid hormone deficiency during late gestation. Proportions of unilocular (UL) (white) and multilocular (ML) (brown) adipocytes, and UL adipocyte size, were assessed by histological and stereological techniques. Changes to the adipose transcriptome were investigated by RNA sequencing and bioinformatic analysis, and proteins of interest were quantified by Western blotting. Results: Hypothyroidism in utero resulted in elevated plasma insulin and leptin concentrations and overgrowth of PAT in the fetus, specifically due to hyperplasia and hypertrophy of UL adipocytes with no change in ML adipocyte mass. RNA sequencing and genomic analyses showed that thyroid deficiency affected 34% of the genes identified in fetal adipose tissue. Enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) pathways were associated with adipogenic, metabolic, and thermoregulatory processes, insulin resistance, and a range of endocrine and adipocytokine signaling pathways. Adipose protein levels of signaling molecules, including phosphorylated S6-kinase (pS6K), glucose transporter isoform 4 (GLUT4), and peroxisome proliferator-activated receptor γ (PPARγ), were increased by fetal hypothyroidism. Fetal thyroid deficiency decreased uncoupling protein 1 (UCP1) protein and mRNA content, and UCP1 thermogenic capacity without any change in ML adipocyte mass. Conclusions: Growth and development of adipose tissue before birth is sensitive to thyroid hormone status in utero. Changes to the adipose transcriptome and phenotype observed in the hypothyroid fetus may have consequences for neonatal survival and the risk of obesity and metabolic dysfunction in later life.
Keywords: adipose; fetus; insulin; insulin-like growth factor; leptin; thyroid hormone; uncoupling protein.
Figures

Similar articles
-
Cold acclimation and pioglitazone combined increase thermogenic capacity of brown and white adipose tissues but this does not translate into higher energy expenditure in mice.Am J Physiol Endocrinol Metab. 2023 Apr 1;324(4):E358-E373. doi: 10.1152/ajpendo.00217.2022. Epub 2023 Mar 1. Am J Physiol Endocrinol Metab. 2023. PMID: 36856189
-
Two key temporally distinguishable molecular and cellular components of white adipose tissue browning during cold acclimation.J Physiol. 2015 Aug 1;593(15):3267-80. doi: 10.1113/JP270805. Epub 2015 Jul 14. J Physiol. 2015. PMID: 26096127 Free PMC article.
-
Theobromine enhances the conversion of white adipocytes into beige adipocytes in a PPARγ activation-dependent manner.J Nutr Biochem. 2022 Feb;100:108898. doi: 10.1016/j.jnutbio.2021.108898. Epub 2021 Nov 5. J Nutr Biochem. 2022. PMID: 34748921
-
The role of leptin in the transition from fetus to neonate.Proc Nutr Soc. 2001 May;60(2):187-94. doi: 10.1079/pns200086. Proc Nutr Soc. 2001. PMID: 11681634 Review.
-
Signaling Pathways Regulating Thermogenesis.Front Endocrinol (Lausanne). 2021 Mar 26;12:595020. doi: 10.3389/fendo.2021.595020. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33841324 Free PMC article. Review.
Cited by
-
Brown to White Fat Transition Overlap With Skeletal Muscle During Development of Larger Mammals: Is it a Coincidence?J Endocr Soc. 2022 Sep 27;6(12):bvac151. doi: 10.1210/jendso/bvac151. eCollection 2022 Oct 26. J Endocr Soc. 2022. PMID: 36325536 Free PMC article. Review.
-
Compensatory mechanisms in response to induced hypothyroidism in the late gestation pig fetus†.Biol Reprod. 2023 May 10;108(5):731-743. doi: 10.1093/biolre/ioad024. Biol Reprod. 2023. PMID: 36811850 Free PMC article.
-
Understanding the Roles of Selenium on Thyroid Hormone-Induced Thermogenesis in Adipose Tissue.Biol Trace Elem Res. 2024 Jun;202(6):2419-2441. doi: 10.1007/s12011-023-03854-2. Epub 2023 Sep 27. Biol Trace Elem Res. 2024. PMID: 37758980 Review.
-
Is Upregulation of Sarcolipin Beneficial or Detrimental to Muscle Function?Front Physiol. 2021 Mar 1;12:633058. doi: 10.3389/fphys.2021.633058. eCollection 2021. Front Physiol. 2021. PMID: 33732165 Free PMC article.
-
Porcine reproductive and respiratory virus 2 infection of the fetus results in multi-organ cell cycle suppression.Vet Res. 2022 Feb 21;53(1):13. doi: 10.1186/s13567-022-01030-3. Vet Res. 2022. PMID: 35189966 Free PMC article.
References
-
- Wassner AJ. Congenital hypothyroidism. Clin Perinatol. 2018;45:1–18. - PubMed
-
- Wong SC, Ng SM, Didi M. Children with congenital hypothyroidism are at risk of adult obesity due to early adiposity rebound. Clin Endocrinol. 2004;61:441–446. - PubMed
-
- Arenz S, Nennstiel-Ratzel U, Wildner M, Dörr HG, von Kries R. Intellectual outcome, motor skills and BMI of children with congenital hypothyroidism: a population-based study. Acta Paediatr. 2008;97:447–450. - PubMed
-
- Léger J, Ecosse E, Roussey M, Lanoë JL, Larroque B. Subtle health impairment and socioeducational attainment in young adult patients with congenital hypothyroidism diagnosed by neonatal screening: a longitudinal population-based cohort study. J Clin Endocrinol Metab. 2011;96:1771–1782. - PubMed
-
- Chen SY, Lin SJ, Lin SH, Chou YY. Early adiposity rebound and obesity in children with congenital hypothyroidism. Pediatr Neonatol. 2013;54:107–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

