Antibacterial activity of iron oxide, iron nitride, and tobramycin conjugated nanoparticles against Pseudomonas aeruginosa biofilms
- PMID: 32070354
- PMCID: PMC7029462
- DOI: 10.1186/s12951-020-0588-6
Antibacterial activity of iron oxide, iron nitride, and tobramycin conjugated nanoparticles against Pseudomonas aeruginosa biofilms
Abstract
Background: Novel methods are necessary to reduce morbidity and mortality of patients suffering from infections with Pseudomonas aeruginosa. Being the most common infectious species of the Pseudomonas genus, P. aeruginosa is the primary Gram-negative etiology responsible for nosocomial infections. Due to the ubiquity and high adaptability of this species, an effective universal treatment method for P. aeruginosa infection still eludes investigators, despite the extensive research in this area.
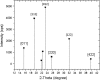
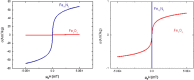
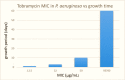
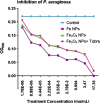
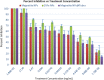
Results: We report bacterial inhibition by iron-oxide (nominally magnetite) nanoparticles (NPs) alone, having a mean hydrodynamic diameter of ~ 16 nm, as well as alginate-capped iron-oxide NPs. Alginate capping increased the average hydrodynamic diameter to ~ 230 nm. We also investigated alginate-capped iron-oxide NP-drug conjugates, with a practically unchanged hydrodynamic diameter of ~ 232 nm. Susceptibility and minimum inhibitory concentration (MIC) of the NPs, NP-tobramycin conjugates, and tobramycin alone were determined in the PAO1 bacterial colonies. Investigations into susceptibility using the disk diffusion method were done after 3 days of biofilm growth and after 60 days of growth. MIC of all compounds of interest was determined after 60-days of growth, to ensure thorough establishment of biofilm colonies.
Conclusions: Positive inhibition is reported for uncapped and alginate-capped iron-oxide NPs, and the corresponding MICs are presented. We report zero susceptibility to iron-oxide NPs capped with polyethylene glycol, suggesting that the capping agent plays a major role in enabling bactericidal ability in of the nanocomposite. Our findings suggest that the alginate-coated nanocomposites investigated in this study have the potential to overcome the bacterial biofilm barrier. Magnetic field application increases the action, likely via enhanced diffusion of the iron-oxide NPs and NP-drug conjugates through mucin and alginate barriers, which are characteristic of cystic-fibrosis respiratory infections. We demonstrate that iron-oxide NPs coated with alginate, as well as alginate-coated magnetite-tobramycin conjugates inhibit P. aeruginosa growth and biofilm formation in established colonies. We have also determined that susceptibility to tobramycin decreases for longer culture times. However, susceptibility to the iron-oxide NP compounds did not demonstrate any comparable decrease with increasing culture time. These findings imply that iron-oxide NPs are promising lower-cost alternatives to silver NPs in antibacterial coatings, solutions, and drugs, as well as other applications in which microbial abolition or infestation prevention is sought.
Keywords: Alginate; Antibacterial agents; Antibiotic resistance; Biofilm; Cystic fibrosis; Drug delivery; Iron-oxide nanoparticles; Magnetite; Pseudomonas aeruginosa; Zero-valent iron nanoparticles.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures













References
-
- Bradford PA, Bratu S, Urban C, Visalli M, Mariano N, Landman D, Rahal JJ, Brooks S, Cebular S, Quale J. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 β-lactamases in New York City. Clin Infect Dis. 2004;39(1):55–60. doi: 10.1086/421495. - DOI - PubMed
-
- Cardo D, Horan T, Andrus M, Dembinski M, Edwards J, Peavy G, Tolson J, Wagner D. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32(8):470–485. doi: 10.1016/j.ajic.2004.10.001. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

