The use of sodium DL-3-Hydroxybutyrate in severe acute neuro-metabolic compromise in patients with inherited ketone body synthetic disorders
- PMID: 32070364
- PMCID: PMC7029565
- DOI: 10.1186/s13023-020-1316-x
The use of sodium DL-3-Hydroxybutyrate in severe acute neuro-metabolic compromise in patients with inherited ketone body synthetic disorders
Abstract
Background: Ketone bodies form a vital energy source for end organs in a variety of physiological circumstances. At different times, the heart, brain and skeletal muscle in particular can use ketones as a primary substrate. Failure to generate ketones in such circumstances leads to compromised energy delivery, critical end-organ dysfunction and potentially death. There are a range of inborn errors of metabolism (IEM) affecting ketone body production that can present in this way, including disorders of carnitine transport into the mitochondrion, mitochondrial fatty acid oxidation deficiencies (MFAOD) and ketone body synthesis. In situations of acute energy deficit, management of IEM typically entails circumventing the enzyme deficiency with replenishment of energy requirements. Due to profound multi-organ failure it is often difficult to provide optimal enteral therapy in such situations and rescue with sodium DL-3-hydroxybutyrate (S DL-3-OHB) has been attempted in these conditions as documented in this paper.
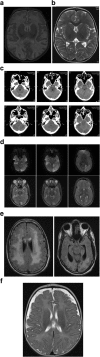
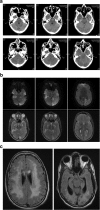
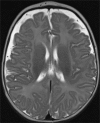
Results: We present 3 cases of metabolic decompensation, one with carnitine-acyl-carnitine translocase deficiency (CACTD) another with 3-hydroxyl, 3-methyl, glutaryl CoA lyase deficiency (HMGCLD) and a third with carnitine palmitoyl transferase II deficiency (CPT2D). All of these disorders are frequently associated with death in circumstance where catastrophic acute metabolic deterioration occurs. Intensive therapy with adjunctive S DL-3OHB led to rapid and sustained recovery in all. Alternative therapies are scarce in these situations.
Conclusion: S DL-3-OHB has been utilised in multiple acyl co A dehydrogenase deficiency (MADD) in cases with acute neurological and cardiac compromise with long-term data awaiting publication. The use of S DL-3-OHB is novel in non-MADD fat oxidation disorders and contribute to the argument for more widespread use.
Keywords: 3-hydroxybutyrate; 3-hydroxyl-3-methylglutaryl-CoA lyase deficiency (HMGCLD); Carnitine acyl-carnitine translocase deficiency (CACTD); Carnitine palmitoyl transferase II deficiency (CPT2D); Fat oxidation; Ketone body.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Berg JMTJ, Stryer L. Biochemistry. New York: W H Freeman; 2005.
-
- Al-Sannaa NA, Cheriyan GM. Carnitine-acylcarnitine translocase deficiency. Clinical course of three Saudi children with a severe phenotype. Saudi Med J. 2010;31(8):931–934. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

