An Individual Participant Data Population Pharmacokinetic Meta-analysis of Drug-Drug Interactions between Lumefantrine and Commonly Used Antiretroviral Treatment
- PMID: 32071050
- PMCID: PMC7179577
- DOI: 10.1128/AAC.02394-19
An Individual Participant Data Population Pharmacokinetic Meta-analysis of Drug-Drug Interactions between Lumefantrine and Commonly Used Antiretroviral Treatment
Abstract
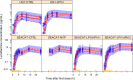
Treating malaria in HIV-coinfected individuals should consider potential drug-drug interactions. Artemether-lumefantrine is the most widely recommended treatment for uncomplicated malaria globally. Lumefantrine is metabolized by CYP3A4, an enzyme that commonly used antiretrovirals often induce or inhibit. A population pharmacokinetic meta-analysis was conducted using individual participant data from 10 studies with 6,100 lumefantrine concentrations from 793 nonpregnant adult participants (41% HIV-malaria-coinfected, 36% malaria-infected, 20% HIV-infected, and 3% healthy volunteers). Lumefantrine exposure increased 3.4-fold with coadministration of lopinavir-ritonavir-based antiretroviral therapy (ART), while it decreased by 47% with efavirenz-based ART and by 59% in the patients with rifampin-based antituberculosis treatment. Nevirapine- or dolutegravir-based ART and malaria or HIV infection were not associated with significant effects. Monte Carlo simulations showed that those on concomitant efavirenz or rifampin have 49% and 80% probability of day 7 concentrations <200 ng/ml, respectively, a threshold associated with an increased risk of treatment failure. The risk of achieving subtherapeutic concentrations increases with larger body weight. An extended 5-day and 6-day artemether-lumefantrine regimen is predicted to overcome these drug-drug interactions with efavirenz and rifampin, respectively.
Keywords: drug-drug interactions; human immunodeficiency virus; lumefantrine; population pharmacokinetics; uncomplicated malaria.
Copyright © 2020 Francis et al.
Figures



References
-
- World Health Organization. 2018. World malaria report. World Health Organization, Geneva, Switzerland.
-
- Ashley EA, Stepniewska K, Lindegårdh N, McGready R, Annerberg A, Hutagalung R, Singtoroj T, Hla G, Brockman A, Proux S, Wilahphaingern J, Singhasivanon P, White NJ, Nosten F. 2007. Pharmacokinetic study of artemether-lumefantrine given once daily for the treatment of uncomplicated multidrug-resistant falciparum malaria. Trop Med Int Health 12:201–208. doi:10.1111/j.1365-3156.2006.01785.x. - DOI - PubMed
-
- Wong RPM, Salman S, Ilett KF, Siba PM, Mueller I, Davis T. 2011. Desbutyl-lumefantrine is a metabolite of lumefantrine with potent in vitro antimalarial activity that may influence artemether-lumefantrine treatment outcome. Antimicrob Agents Chemother 55:1194–1198. doi:10.1128/AAC.01312-10. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

