Abacavir Exposure in Children Cotreated for Tuberculosis with Rifampin and Superboosted Lopinavir-Ritonavir
- PMID: 32071055
- PMCID: PMC7179606
- DOI: 10.1128/AAC.01923-19
Abacavir Exposure in Children Cotreated for Tuberculosis with Rifampin and Superboosted Lopinavir-Ritonavir
Abstract
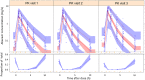
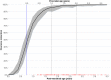
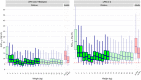
In children requiring lopinavir coformulated with ritonavir in a 4:1 ratio (lopinavir-ritonavir-4:1) and rifampin, adding ritonavir to achieve a 4:4 ratio with lopinavir (LPV/r-4:4) overcomes the drug-drug interaction. Possible drug-drug interactions within this regimen may affect abacavir concentrations, but this has never been studied. Children weighing <15 kg needing rifampin and LPV/r-4:4 were enrolled in a pharmacokinetic study and underwent intensive pharmacokinetic sampling on 3 visits: (i) during the intensive and (ii) continuation phases of antituberculosis treatment with LPV/r-4:4 and (iii) 1 month after antituberculosis treatment completion on LPV/r-4:1. Pharmacometric modeling and simulation were used to compare exposures across weight bands with adult target exposures. Eighty-seven children with a median (interquartile range) age and weight of 19 (4 to 64) months and 8.7 (3.9 to 14.9) kg, respectively, were included in the abacavir analysis. Abacavir pharmacokinetics were best described by a two-compartment model with first-order elimination and transit compartment absorption. After allometric scaling adjusted for the effect of body size, maturation could be identified: clearance was predicted to be fully mature at about 2 years of age and to reach half of this mature value at about 2 months of age. Abacavir bioavailability decreased 36% during treatment with rifampin and LPV/r-4:4 but remained within the median adult recommended exposure, except for children in the 3- to 4.9-kg weight band, in which the exposures were higher. The observed predose morning trough concentrations were higher than the evening values. Though abacavir exposure significantly decreased during concomitant administration of rifampin and LPV/r-4:4, it remained within acceptable ranges. (This study is registered in ClinicalTrials.gov under identifier NCT02348177.).
Keywords: NONMEM; abacavir; children; lopinavir; population pharmacokinetics; rifampin.
Copyright © 2020 Rabie et al.
Figures

References
-
- World Health Organization. 2019. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV. World Health Organization, Geneva, Switzerland.
-
- Bergshoeff A, Burger D, Verweij C, Farrelly L, Flynn J, Le Prevost M, Walker S, Novelli V, Lyall H, Khoo S, Gibb D, PENTA-13 Study Group. 2005. Plasma pharmacokinetics of once- versus twice-daily lamivudine and abacavir: simplification of combination treatment in HIV-1-infected children (PENTA-13). Antivir Ther 10:239–246. - PubMed
-
- Western Cape Health Department. 2018. The Western Cape consolidated guidelines for HIV treatment: prevention of mother- to- child transmission of HIV (PMTCT), children, adolescents and adults (amended version). Western Cape Health Department, Cape Town, South Africa.
-
- Nahirya-Ntege P, Musiime V, Naidoo B, Bakeera-Kitaka S, Nathoo K, Munderi P, Mugyenyi P, Kekitiinwa A, Bwakura-Dangarembizi MF, Crawley J, ARROW Trial Team. 2011. Low incidence of abacavir hypersensitivity reaction among African children initiating antiretroviral therapy. Pediatr Infect Dis J 30:535–537. doi:10.1097/INF.0b013e3182076864. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

