Results of a Randomized Phase IIb Trial of Nelipepimut-S + Trastuzumab versus Trastuzumab to Prevent Recurrences in Patients with High-Risk HER2 Low-Expressing Breast Cancer
- PMID: 32071118
- PMCID: PMC8771534
- DOI: 10.1158/1078-0432.CCR-19-2741
Results of a Randomized Phase IIb Trial of Nelipepimut-S + Trastuzumab versus Trastuzumab to Prevent Recurrences in Patients with High-Risk HER2 Low-Expressing Breast Cancer
Abstract
Purpose: Preclinical data provide evidence for synergism between HER2-targeted peptide vaccines and trastuzumab. The efficacy of this combination was evaluated in patients with HER2 low-expressing breast cancer in the adjuvant setting.
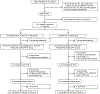
Patients and methods: A phase IIb, multicenter, randomized, single-blinded, controlled trial enrolled disease-free patients after standard therapy completion (NCT01570036). Eligible patients were HLA-A2, A3, A24, and/or A26+, and had HER2 IHC 1+/2+, FISH nonamplified breast cancer, that was node positive and/or hormone receptor-negative [triple-negative breast cancer (TNBC)]. Patients received trastuzumab for 1 year and were randomized to placebo (GM-CSF, control) or nelipepimut-S (NPS) with GM-CSF. Primary outcome was 24-month disease-free survival (DFS). Secondary outcomes were 36-month DFS, safety, and immunologic response.
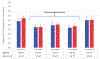
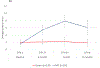
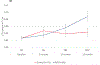
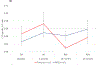
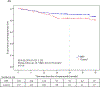
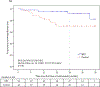
Results: Overall, 275 patients were randomized; 136 received NPS with GM-CSF, and 139 received placebo with GM-CSF. There were no clinicopathologic differences between groups. Concurrent trastuzumab and NPS with GM-CSF was safe with no additional overall or cardiac toxicity compared with control. At median follow-up of 25.7 (interquartile range, 18.4-32.7) months, estimated DFS did not significantly differ between NPS and control [HR, 0.62; 95% confidence interval (CI), 0.31-1.25; P = 0.18]. In a planned exploratory analysis of patients with TNBC, DFS was improved for NPS versus control (HR, 0.26; 95% CI, 0.08-0.81, P = 0.01).
Conclusions: The combination of NPS with trastuzumab is safe. In HER2 low-expressing breast cancer, no significant difference in DFS was seen in the intention-to-treat analysis; however, significant clinical benefit was seen in patients with TNBC. These findings warrant further investigation in a phase III randomized trial.
©2020 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest Statement:
Dr. Litton reports grant or research support from Novartis, Medivation/Pfizer, Genentech, GSK, EMD-Serono, Astra-Zeneca, and Medimmune; Speaker’s Bureau for MedLearning, Physician’s Education Resource, Prime Oncology, Medscape, and Clinical Care Options; employment at The University of Texas MD Anderson Cancer Center; honoraria from UpToDate; advisory committee/review panel/board membership for Astra-Zeneca and Pfizer (both uncompensated); and review panels for NCCN, ASCO, and NIH PDQ. Dr. Murthy reports consulting fees for participation on advisory boards for Daiichi Sankyo, Genentech and Puma; honorarium from France Foundation for non-CME services; and research support (to institution) from EMD-Serono, Pfizer, Seattle Genetics, Daiichi Sankyo, and Genentech. Dr. Peoples reports grants from Sellas Life Sciences, during the conduct of the study. In addition, Dr. Peoples has a patent to Nelipepimut-S issued. Dr. Mittendorf reports receiving compensation for participating on Scientific Advisory Boards for Astra-Zeneca, Genentech, Genomic Health, Merck, and Sellas Life Sciences.
The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, The US Army Medical Department, the US Army Office of the Surgeon General, the Department of the Army, Department of Defense, or the US Government.
Figures





References
-
- Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, Geyer CE Jr., et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol 2011;29:3366–73. - PMC - PubMed
-
- Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr., et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 2014;32:3744–52. - PMC - PubMed
-
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr., Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673–84. - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244:707–12. - PubMed
-
- Fehrenbacher L, Cecchini RS, Geyer CE, Rastogi P, Costantino JP. NSABP B-47 (NRG oncology): Phase III randomized trial comparing adjuvant chemotherapy with adriamycin (A) and cyclophosphamide (C) → weekly paclitaxel (WP), or docetaxel (T) and C with or without a year of trastuzumab (H) in women with node-positive or high-risk node-negative invasive breast cancer (IBC) expressing HER2 staining intensity of IHC 1+ or 2+ with negative FISH (HER2-Low IBC) [Abstract]. In: Proceedings of the 2017 San Antonio Breast Cancer Symposium; Dec 5–9; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2018;78(4 Suppl):Abstract nr GS1–02.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

