Open-label placebo treatment of women with premenstrual syndrome: study protocol of a randomised controlled trial
- PMID: 32071176
- PMCID: PMC7045079
- DOI: 10.1136/bmjopen-2019-032868
Open-label placebo treatment of women with premenstrual syndrome: study protocol of a randomised controlled trial
Abstract
Introduction: Recent evidence suggests that for certain clinical conditions, placebos can improve clinical outcomes even without deception. These so-called open-label placebos (OLPs) bear the advantage of a significant lower risk of adverse events and comply with ethical principles. Although premenstrual syndrome (PMS) seems to be considerably susceptible to placebo effects, no study has examined open-OLP responses on PMS.
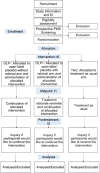
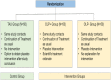
Methods and analysis: To test the efficacy of OLPs in women suffering from PMS, a clinical randomised controlled trial including two OLP study groups (with and without treatment rationale) was designed to investigate on the effect on PMS. PMS symptoms are monitored on a daily basis via a symptom diary, adverse events are monitored intermittently. The study started in spring 2018 and patients will be included until a maximum of 150 participants are randomised. Besides the primary outcome PMS symptom intensity and interference, an array of further variables is assessed. Multilevel modelling will be used for data analyses.
Ethics and dissemination: Ethics approval was obtained from the Ethics Committee Northwest and Central Switzerland. Results of the main analysis and of secondary analyses will be submitted for publication in peer-reviewed journals. TRIAL REGISTRATION NUMBERS: (1) ClinicalTrials.gov (NCT03547661); (2) Swiss national registration (SNCTP000002809).
Keywords: PMS symptom diary; open-label placebos; premenstrual syndrome; randomised controlled trial; treatment rationale.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical