Challenges for funders in monitoring compliance with policies on clinical trials registration and reporting: analysis of funding and registry data in the UK
- PMID: 32071191
- PMCID: PMC7045207
- DOI: 10.1136/bmjopen-2019-035283
Challenges for funders in monitoring compliance with policies on clinical trials registration and reporting: analysis of funding and registry data in the UK
Abstract
Objectives: To evaluate compliance by researchers with funder requirements on clinical trial transparency, including identifying key areas for improvement; to assess the completeness, accuracy and suitability for annual compliance monitoring of the data routinely collected by a research funding body.
Design: Descriptive analysis of clinical trials funded between February 2011 and January 2017 against funder policy requirements.
Setting: Public medical research funding body in the UK.
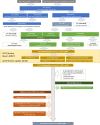
Data sources: Relevant clinical trials were identified from grant application details, post-award grant monitoring systems and the International Standard Randomised Controlled Trial Number (ISRCTN) registry.
Main outcome measure: The proportion of all Medical Research Council (MRC)-funded clinical trials that were (a) registered in a clinical trial registry and (b) publicly reported summary results within 2 years of completion.
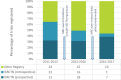
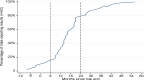
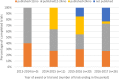
Results: There were 175 grants awarded that included a clinical trial and all trials were registered in a public trials registry. Of 62 trials completed for over 24 months, 42 (68%) had publicly reported the main findings by 24 months after trial completion; 18 of these achieved this within 12 months of completion. 11 (18%) trials took >24 months to report and 9 (15%) completed trials had not yet reported findings. Five datasets were shared with other researchers.
Conclusions: Compliance with the funder policy requirements on trial registration was excellent. Reporting of the main findings was achieved for most trials within 24 months of completion; however, the number of unreported trials remains a concern and should be a focus for future funder policy initiatives. Identifying trials from grant management and grant monitoring systems was challenging therefore funders should ensure investigators reliably provide trial registries with information and regularly update entries with details of trial publications and protocols.
Keywords: audit; clinical trials; statistics & research methods.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Adoption of World Health Organization Best Practices in Clinical Trial Transparency Among European Medical Research Funder Policies.JAMA Netw Open. 2022 Aug 1;5(8):e2222378. doi: 10.1001/jamanetworkopen.2022.22378. JAMA Netw Open. 2022. PMID: 35913742 Free PMC article.
-
Noncommercial US Funders' Policies on Trial Registration, Access to Summary Results, and Individual Patient Data Availability.JAMA Netw Open. 2019 Jan 4;2(1):e187498. doi: 10.1001/jamanetworkopen.2018.7498. JAMA Netw Open. 2019. PMID: 30681715 Free PMC article.
-
Clinical trial reporting performance of thirty UK universities on ClinicalTrials.gov-evaluation of a new tracking tool for the US clinical trial registry.Trials. 2021 Jun 1;22(1):375. doi: 10.1186/s13063-021-05330-5. Trials. 2021. PMID: 34074329 Free PMC article.
-
An audit to evaluate an institute's lead researchers' knowledge of trial registries and to investigate adherence to data transparency issues in an Italian research institute registry.Trials. 2018 Sep 20;19(1):509. doi: 10.1186/s13063-018-2910-2. Trials. 2018. PMID: 30236146 Free PMC article. Review.
-
A reinvestigation of recruitment to randomised, controlled, multicenter trials: a review of trials funded by two UK funding agencies.Trials. 2013 Jun 9;14:166. doi: 10.1186/1745-6215-14-166. Trials. 2013. PMID: 23758961 Free PMC article. Review.
Cited by
-
Practices and trends in clinical trial registration in the Pan African Clinical Trials Registry (PACTR): a descriptive analysis of registration data.BMJ Open. 2022 Jan 25;12(1):e057474. doi: 10.1136/bmjopen-2021-057474. BMJ Open. 2022. PMID: 35078852 Free PMC article.
-
How an international research funder's forum developed guiding principles to ensure value and reduce waste in research.F1000Res. 2023 Dec 28;12:310. doi: 10.12688/f1000research.128797.2. eCollection 2023. F1000Res. 2023. PMID: 38845618 Free PMC article.
-
Compliance of Clinical Trial Protocols for Foods with Function Claims (FFC) in Japan: Consistency between Clinical Trial Registrations and Published Reports.Nutrients. 2021 Dec 25;14(1):81. doi: 10.3390/nu14010081. Nutrients. 2021. PMID: 35010956 Free PMC article. Review.
-
Missing clinical trial data: the evidence gap in primary data for potential COVID-19 drugs.Trials. 2021 Jan 15;22(1):59. doi: 10.1186/s13063-021-05024-y. Trials. 2021. PMID: 33451350 Free PMC article.
-
Adoption of World Health Organization Best Practices in Clinical Trial Transparency Among European Medical Research Funder Policies.JAMA Netw Open. 2022 Aug 1;5(8):e2222378. doi: 10.1001/jamanetworkopen.2022.22378. JAMA Netw Open. 2022. PMID: 35913742 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
