Spatial mapping of tissue properties in vivo reveals a 3D stiffness gradient in the mouse limb bud
- PMID: 32071242
- PMCID: PMC7060671
- DOI: 10.1073/pnas.1912656117
Spatial mapping of tissue properties in vivo reveals a 3D stiffness gradient in the mouse limb bud
Abstract
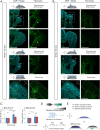
Numerous hypotheses invoke tissue stiffness as a key parameter that regulates morphogenesis and disease progression. However, current methods are insufficient to test hypotheses that concern physical properties deep in living tissues. Here we introduce, validate, and apply a magnetic device that generates a uniform magnetic field gradient within a space that is sufficient to accommodate an organ-stage mouse embryo under live conditions. The method allows rapid, nontoxic measurement of the three-dimensional (3D) spatial distribution of viscoelastic properties within mesenchyme and epithelia. Using the device, we identify an anteriorly biased mesodermal stiffness gradient along which cells move to shape the early limb bud. The stiffness gradient corresponds to a Wnt5a-dependent domain of fibronectin expression, raising the possibility that durotaxis underlies cell movements. Three-dimensional stiffness mapping enables the generation of hypotheses and potentially the rigorous testing of mechanisms of development and disease.
Keywords: cell movements; durotaxis; limb bud; morphogenesis; tissue stiffness.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

