Phage liquid crystalline droplets form occlusive sheaths that encapsulate and protect infectious rod-shaped bacteria
- PMID: 32071243
- PMCID: PMC7060675
- DOI: 10.1073/pnas.1917726117
Phage liquid crystalline droplets form occlusive sheaths that encapsulate and protect infectious rod-shaped bacteria
Abstract
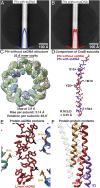
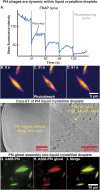
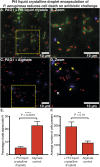
The opportunistic pathogen Pseudomonas aeruginosa is a major cause of antibiotic-tolerant infections in humans. P. aeruginosa evades antibiotics in bacterial biofilms by up-regulating expression of a symbiotic filamentous inoviral prophage, Pf4. We investigated the mechanism of phage-mediated antibiotic tolerance using biochemical reconstitution combined with structural biology and high-resolution cellular imaging. We resolved electron cryomicroscopy atomic structures of Pf4 with and without its linear single-stranded DNA genome, and studied Pf4 assembly into liquid crystalline droplets using optical microscopy and electron cryotomography. By biochemically replicating conditions necessary for antibiotic protection, we found that phage liquid crystalline droplets form phase-separated occlusive compartments around rod-shaped bacteria leading to increased bacterial survival. Encapsulation by these compartments was observed even when inanimate colloidal rods were used to mimic rod-shaped bacteria, suggesting that shape and size complementarity profoundly influences the process. Filamentous inoviruses are pervasive across prokaryotes, and in particular, several Gram-negative bacterial pathogens including Neisseria meningitidis, Vibrio cholerae, and Salmonella enterica harbor these prophages. We propose that biophysical occlusion mediated by secreted filamentous molecules such as Pf4 may be a general strategy of bacterial survival in harsh environments.
Keywords: Pseudomonas aeruginosa; antibiotic tolerance; cryo-EM; phage; phase separation.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures




Comment in
-
Phage liquid crystals protect Pseudomonas.Nat Rev Microbiol. 2020 May;18(5):264-265. doi: 10.1038/s41579-020-0347-6. Nat Rev Microbiol. 2020. PMID: 32099077 No abstract available.
-
Bacteria suit up with virus armor.Proc Natl Acad Sci U S A. 2020 Mar 24;117(12):6297-6299. doi: 10.1073/pnas.2001931117. Epub 2020 Mar 9. Proc Natl Acad Sci U S A. 2020. PMID: 32152106 Free PMC article. No abstract available.
References
-
- Costerton J. W., Stewart P. S., Greenberg E. P., Bacterial biofilms: A common cause of persistent infections. Science 284, 1318–1322 (1999). - PubMed
-
- Hall-Stoodley L., Stoodley P., Evolving concepts in biofilm infections. Cell. Microbiol. 11, 1034–1043 (2009). - PubMed
-
- Høiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O., Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 35, 322–332 (2010). - PubMed
-
- Whiteley M., et al. , Gene expression in Pseudomonas aeruginosa biofilms. Nature 413, 860–864 (2001). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

