The microbiome and gynaecological cancer development, prevention and therapy
- PMID: 32071434
- PMCID: PMC9977514
- DOI: 10.1038/s41585-020-0286-z
The microbiome and gynaecological cancer development, prevention and therapy
Abstract
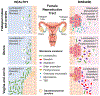
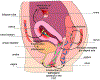
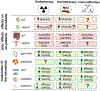
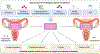
The female reproductive tract (FRT), similar to other mucosal sites, harbours a site-specific microbiome, which has an essential role in maintaining health and homeostasis. In the majority of women of reproductive age, the microbiota of the lower FRT (vagina and cervix) microenvironment is dominated by Lactobacillus species, which benefit the host through symbiotic relationships. By contrast, the upper FRT (uterus, Fallopian tubes and ovaries) might be sterile in healthy individuals or contain a low-biomass microbiome with a diverse mixture of microorganisms. When dysbiosis occurs, altered immune and metabolic signalling can affect hallmarks of cancer, including chronic inflammation, epithelial barrier breach, changes in cellular proliferation and apoptosis, genome instability, angiogenesis and metabolic dysregulation. These pathophysiological changes might lead to gynaecological cancer. Emerging evidence shows that genital dysbiosis and/or specific bacteria might have an active role in the development and/or progression and metastasis of gynaecological malignancies, such as cervical, endometrial and ovarian cancers, through direct and indirect mechanisms, including modulation of oestrogen metabolism. Cancer therapies might also alter microbiota at sites throughout the body. Reciprocally, microbiota composition can influence the efficacy and toxic effects of cancer therapies, as well as quality of life following cancer treatment. Modulation of the microbiome via probiotics or microbiota transplant might prove useful in improving responsiveness to cancer treatment and quality of life. Elucidating these complex host-microbiome interactions, including the crosstalk between distal and local sites, will translate into interventions for prevention, therapeutic efficacy and toxic effects to enhance health outcomes for women with gynaecological cancers.
Conflict of interest statement
Competing interests
M.M.H.-K. has been a consultant for Lupin Pharmaceuticals and Beckton Dickinson. P.Ł. and Z.E.I. declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

