Preparation of the standard cell lines for reference mutations in cancer gene-panels by genome editing in HEK 293 T/17 cells
- PMID: 32071619
- PMCID: PMC7014756
- DOI: 10.1186/s41021-020-0147-2
Preparation of the standard cell lines for reference mutations in cancer gene-panels by genome editing in HEK 293 T/17 cells
Abstract
Background: Next Generation Sequencer (NGS) is a powerful tool for a high-throughput sequencing of human genome. It is important to ensure reliability and sensitivity of the sequence data for a clinical use of the NGS. Various cancer-related gene panels such as Oncomine™ or NCC OncoPanel have been developed and used for clinical studies. Because these panels contain multiple genes, it is difficult to ensure the performance of mutation detection for every gene. In addition, various platforms of NGS are developed and their cross-platform validation has become necessity. In order to create mutant standards in a defined background, we have used CRISPR/Cas9 genome-editing system in HEK 293 T/17 cells.
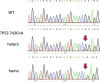
Results: Cancer-related genes that are frequently used in NGS-based cancer panels were selected as the target genes. Target mutations were selected based on their frequency reported in database, and clinical significance and on the applicability of CRISPR/Cas9 by considering distance from PAM site, and off-targets. We have successfully generated 88 hetero- and homozygous mutant cell lines at the targeted sites of 36 genes representing a total of 125 mutations.
Conclusions: These knock-in HEK293T/17 cells can be used as the reference mutant standards with a steady and continuous supply for NGS-based cancer panel tests from the JCRB cell bank. In addition, these cell lines can provide a tool for the functional analysis of targeted mutations in cancer-related genes in the isogenic background.
Keywords: CRISPR/Cas9; Cancer gene panel; Genome editing; Mutant standard; Next generation sequencer.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures

References
-
- Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet. 2010;11(10):685–696. - PubMed
-
- Wakai T, Prasoon P, Hirose Y, Shimada Y, Ichikawa H, Nagahashi M. Next-generation sequencing-based clinical sequencing: toward precision medicine in solid tumors. Int J Clin Oncol. 2019;24(2):115–122. - PubMed
-
- Schwartzberg L, Kim ES, Liu D, Schrag D. Precision oncology: who, how, what, when, and when not? Am Soc Clin Oncol Educ Book. 2017;37:160–169. - PubMed
-
- Sabour L, Sabour M, Ghorbian S. Clinical applications of next-generation sequencing in Cancer diagnosis. Pathol Oncol Res. 2017;23(2):225–234. - PubMed
-
- Kumar B, Singh S, Skvortsova I, Kumar V. Promising targets in anti-cancer drug development: recent updates. Curr Med Chem. 2017;24(42):4729–4752. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

