Vorasidenib (AG-881): A First-in-Class, Brain-Penetrant Dual Inhibitor of Mutant IDH1 and 2 for Treatment of Glioma
- PMID: 32071674
- PMCID: PMC7025383
- DOI: 10.1021/acsmedchemlett.9b00509
Vorasidenib (AG-881): A First-in-Class, Brain-Penetrant Dual Inhibitor of Mutant IDH1 and 2 for Treatment of Glioma
Abstract
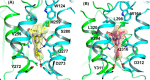
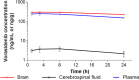
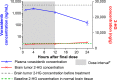
Inhibitors of mutant isocitrate dehydrogenase (mIDH) 1 and 2 cancer-associated enzymes prevent the accumulation of the oncometabolite d-2-hydroxyglutarate (2-HG) and are under clinical investigation for the treatment of several cancers harboring an IDH mutation. Herein, we describe the discovery of vorasidenib (AG-881), a potent, oral, brain-penetrant dual inhibitor of both mIDH1 and mIDH2. X-ray cocrystal structures allowed us to characterize the compound binding site, leading to an understanding of the dual mutant inhibition. Furthermore, vorasidenib penetrates the brain of several preclinical species and inhibits 2-HG production in glioma tissue by >97% in an orthotopic glioma mouse model. Vorasidenib represents a novel dual mIDH1/2 inhibitor and is currently in clinical development for the treatment of low-grade mIDH glioma.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): All Agios authors were employees and stockholders at the time of the study.
Figures

References
-
- Yan H.; Parsons D. W.; Jin G.; McLendon R.; Rasheed B. A.; Yuan W.; Kos I.; Batinic-Haberle I.; Jones S.; Riggins G. J.; Friedman H.; Friedman A.; Reardon D.; Herndon J.; Kinzler K. W.; Velculescu V. E.; Vogelstein B.; Bigner D. D. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360 (8), 765–773. 10.1056/NEJMoa0808710. - DOI - PMC - PubMed
-
- Ward P. S.; Patel J.; Wise D. R.; Abdel-Wahab O.; Bennett B. D.; Coller H. A.; Cross J. R.; Fantin V. R.; Hedvat C. V.; Perl A. E.; Rabinowitz J. D.; Carroll M.; Su S. M.; Sharp K. A.; Levine R. L.; Thompson C. B. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 2010, 17 (3), 225–234. 10.1016/j.ccr.2010.01.020. - DOI - PMC - PubMed
-
- Parsons D. W.; Jones S.; Zhang X.; Lin J. C.; Leary R. J.; Angenendt P.; Mankoo P.; Carter H.; Siu I. M.; Gallia G. L.; Olivi A.; McLendon R.; Rasheed B. A.; Keir S.; Nikolskaya T.; Nikolsky Y.; Busam D. A.; Tekleab H.; Diaz L. A. Jr.; Hartigan J.; Smith D. R.; Strausberg R. L.; Marie S. K.; Shinjo S. M.; Yan H.; Riggins G. J.; Bigner D. D.; Karchin R.; Papadopoulos N.; Parmigiani G.; Vogelstein B.; Velculescu V. E.; Kinzler K. W. An integrated genomic analysis of human glioblastoma multiforme. Science 2008, 321 (5897), 1807–1812. 10.1126/science.1164382. - DOI - PMC - PubMed
-
- Borger D. R.; Tanabe K. K.; Fan K. C.; Lopez H. U.; Fantin V. R.; Straley K. S.; Schenkein D. P.; Hezel A. F.; Ancukiewicz M.; Liebman H. M.; Kwak E. L.; Clark J. W.; Ryan D. P.; Deshpande V.; Dias-Santagata D.; Ellisen L. W.; Zhu A. X.; Iafrate A. J. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 2012, 17 (1), 72–79. 10.1634/theoncologist.2011-0386. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Miscellaneous

