Involvement of five catalytically active Arabidopsis β-amylases in leaf starch metabolism and plant growth
- PMID: 32072133
- PMCID: PMC7011640
- DOI: 10.1002/pld3.199
Involvement of five catalytically active Arabidopsis β-amylases in leaf starch metabolism and plant growth
Abstract
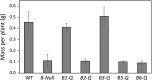
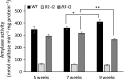
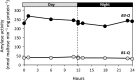
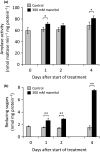
Starch degradation in chloroplasts requires β-amylase (BAM) activity, but in Arabidopsis, there are nine BAM proteins, five of which are thought to be catalytic. Although single-gene knockouts revealed the necessity of BAM3 for starch degradation, contributions of other BAMs are poorly understood. Moreover, it is not possible to detect the contribution of individual BAMs in plants containing multiple active BAMs. Therefore, we constructed a set of five quadruple mutants each expressing only one catalytically active BAM, and a quintuple mutant missing all of these BAMs (B-Null). Using these mutants, we assessed the influence of each individual BAM on plant growth and on leaf starch degradation. Both BAM1 and BAM3 alone support wild-type (WT) levels of growth. BAM3 alone is sufficient to degrade leaf starch completely whereas BAM1 alone can only partially degrade leaf starch. In contrast, BAM2, BAM5, and BAM6 have no detectable effect on starch degradation or plant growth, being comparable with the B-Null plants. B-Null plant extracts contained no measurable amylase activity, whereas BAM3 and BAM1 contributed about 70% and 14% of the WT activity, respectively. BAM2 activity was low but detectable and BAM6 contributed no measurable activity. Interestingly, activity of BAM1 and BAM3 in the mutants varied little developmentally or diurnally, and did not increase appreciably in response to osmotic or cold stress. With these genetic lines, we now have new opportunities to investigate members of this diverse gene family.
Keywords: cold stress; osmotic stress; starch; starch degradation; β‐amylase.
© 2020 The Authors. Plant Direct published by American Society of Plant Biologists and the Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest associated with the work described in this manuscript.
Figures



References
-
- Baunsgaard, L. , Lütken, H. , Mikkelsen, R. , Glaring, M. A. , Pham, T. T. , & Blennow, A. (2005). A novel isoform of glucan, water dikinase phosphorylates pre‐phosphorylated alpha‐glucans and is involved in starch degradation in Arabidopsis. The Plant Journal, 41, 595–605. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

