Application of the 2019 ESC/EAS dyslipidaemia guidelines to nationwide data of patients with a recent myocardial infarction: a simulation study
- PMID: 32072178
- PMCID: PMC7654933
- DOI: 10.1093/eurheartj/ehaa034
Application of the 2019 ESC/EAS dyslipidaemia guidelines to nationwide data of patients with a recent myocardial infarction: a simulation study
Abstract
Aims: To estimate the proportion of patients with a recent myocardial infarction (MI) who would be eligible for additional lipid-lowering therapy according to the 2019 European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) guidelines for the management of dyslipidaemias, and to simulate the effects of expanded lipid-lowering therapy on attainment of the low-density lipoprotein cholesterol (LDL-C) target as recommended by the guidelines.
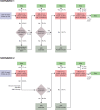
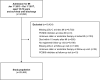
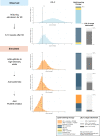
Methods and results: Using the nationwide SWEDEHEART register, we included 25 466 patients who had attended a follow-up visit 6-10 weeks after an MI event, 2013-17. While most patients (86.6%) were receiving high-intensity statins, 82.9% of the patients would be eligible for expanded lipid-lowering therapy, as they had not attained the target of an LDL-C level of <1.4 mmol and a ≥50% LDL-C level reduction. When maximized use of high-intensity statins followed by add-on therapy with ezetimibe was simulated using a Monte Carlo model, the LDL-C target was reached in 19.9% using high-intensity statin monotherapy and in another 28.5% with high-intensity statins and ezetimibe, while 50.7% would still be eligible for proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. When use of alirocumab or evolocumab was simulated in those who were eligible for PCSK9 inhibitors, around 90% of all patients attained the LDL-C target.
Conclusion: Our study suggests that, even with maximized use of high-intensity statins and ezetimibe, around half of patients with MI would be eligible for treatment with PCSK9 inhibitors according to the 2019 ESC/EAS guidelines. Considering the current cost of PCSK9 inhibitors, the financial implications of the new guidelines may be substantial.
Keywords: ESC/EAS guidelines; Ezetimibe; LDL cholesterol target; PCSK9 inhibitors; Statins; Treatment goals.
© The Author(s) 2020. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



Comment in
-
Real-world data, theoretical application of guidelines, cost, and access: how do we optimize non-statin therapy for LDL-C/non-HDL-C/ApoB?Eur Heart J. 2020 Oct 21;41(40):3910-3912. doi: 10.1093/eurheartj/ehaa139. Eur Heart J. 2020. PMID: 32268351 No abstract available.
Similar articles
-
ESC/EAS guidelines for the detection, prevention, and treatment of individuals at risk of a first myocardial infarction: effect of 5 years of updates and the new SCORE2.Eur Heart J Cardiovasc Pharmacother. 2022 Sep 3;8(6):633-643. doi: 10.1093/ehjcvp/pvac021. Eur Heart J Cardiovasc Pharmacother. 2022. PMID: 35381063
-
Low-density lipoprotein-cholesterol target attainment according to the 2011 and 2016 ESC/EAS dyslipidaemia guidelines in patients with a recent myocardial infarction: nationwide cohort study, 2013-17.Eur Heart J Qual Care Clin Outcomes. 2021 Jan 25;7(1):59-67. doi: 10.1093/ehjqcco/qcaa016. Eur Heart J Qual Care Clin Outcomes. 2021. PMID: 32142112 Free PMC article.
-
Eligibility for PCSK9 Inhibitors According to American College of Cardiology (ACC) and European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) Guidelines After Acute Coronary Syndromes.J Am Heart Assoc. 2017 Nov 9;6(11):e006537. doi: 10.1161/JAHA.117.006537. J Am Heart Assoc. 2017. PMID: 29122809 Free PMC article.
-
Statins and PCSK9 inhibitors: A new lipid-lowering therapy.Eur J Pharmacol. 2020 Jul 5;878:173114. doi: 10.1016/j.ejphar.2020.173114. Epub 2020 Apr 14. Eur J Pharmacol. 2020. PMID: 32302598 Review.
-
Recent Updates on the Use of PCSK9 Inhibitors in Patients with Atherosclerotic Cardiovascular Disease.Curr Atheroscler Rep. 2019 Mar 16;21(5):16. doi: 10.1007/s11883-019-0778-6. Curr Atheroscler Rep. 2019. PMID: 30877491 Review.
Cited by
-
Suboptimal management of dyslipidemia in everyday clinical practice: Alarming signals from real-world data.Int J Cardiol. 2020 Oct 1;316:240-241. doi: 10.1016/j.ijcard.2020.06.059. Epub 2020 Jul 4. Int J Cardiol. 2020. PMID: 32634493 Free PMC article. No abstract available.
-
[Cost-benefit analysis of new lipid-lowering agents].Herz. 2022 Jun;47(3):236-243. doi: 10.1007/s00059-022-05116-8. Epub 2022 Apr 25. Herz. 2022. PMID: 35467096 Review. German.
-
[Primary and secondary prevention in hypercholesterolemia: differences relevant to patient care in the PROCYON trial].Dtsch Med Wochenschr. 2023 Sep;148(19):e101-e110. doi: 10.1055/a-2117-6504. Epub 2023 Aug 21. Dtsch Med Wochenschr. 2023. PMID: 37604168 Free PMC article. German.
-
Treatment pathways of lipid-lowering therapies in Germany 2016-2022.Clin Res Cardiol. 2025 May 28. doi: 10.1007/s00392-025-02686-5. Online ahead of print. Clin Res Cardiol. 2025. PMID: 40434561
-
Prevalence and Patient Outcomes of Adult Primary Hypercholesterolemia and Dyslipidemia in the UK: Longitudinal Retrospective Study Using a Primary Care Dataset from 2009 to 2019.Clinicoecon Outcomes Res. 2022 Apr 5;14:189-203. doi: 10.2147/CEOR.S347085. eCollection 2022. Clinicoecon Outcomes Res. 2022. PMID: 35411162 Free PMC article.
References
-
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188. - PubMed
-
- Catapano AL, Graham I, Backer GD, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen M-R, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058. - PubMed
-
- Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart 2010;96:1617–1621. - PubMed
-
- Kereiakes DJ, Robinson JG, Cannon CP, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J 2015;169:906–915.e13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

