Targets for protection and mitigation of radiation injury
- PMID: 32072238
- PMCID: PMC11104832
- DOI: 10.1007/s00018-020-03479-x
Targets for protection and mitigation of radiation injury
Abstract
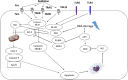
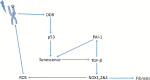
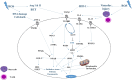
Protection of normal tissues against toxic effects of ionizing radiation is a critical issue in clinical and environmental radiobiology. Investigations in recent decades have suggested potential targets that are involved in the protection against radiation-induced damages to normal tissues and can be proposed for mitigation of radiation injury. Emerging evidences have been shown to be in contrast to an old dogma in radiation biology; a major amount of reactive oxygen species (ROS) production and cell toxicity occur during some hours to years after exposure to ionizing radiation. This can be attributed to upregulation of inflammatory and fibrosis mediators, epigenetic changes and disruption of the normal metabolism of oxygen. In the current review, we explain the cellular and molecular changes following exposure of normal tissues to ionizing radiation. Furthermore, we review potential targets that can be proposed for protection and mitigation of radiation toxicity.
Keywords: Acute radiation syndrome (ARS); Fibrosis; Inflammation; Mitigation; Mitochondria; Normal tissue injury; Pneumonitis; ROS; Radiation; Redox.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures


References
-
- Lindegaard JC, Grau C. Has the outlook improved for amifostine as a clinical radioprotector? Radiother Oncol. 2000;57(2):113–118. - PubMed
-
- Wasserman T. Radioprotective effects of amifostine. Semin Oncol. 1999;20:20. - PubMed
-
- Abt G, Vaghef H, Gebhart E, Dahlgren CV, Hellman B. The role of N-acetylcysteine as a putative radioprotective agent on X-ray-induced DNA damage as evaluated by alkaline single-cell gel electrophoresis. Mutation Res DNA Rep. 1997;384(1):55–64. - PubMed
-
- Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, Monnier A, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18(19):3339–3345. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

