Different mechanisms for modulation of the initiation and steady-state of smooth pursuit eye movements
- PMID: 32073944
- PMCID: PMC7099477
- DOI: 10.1152/jn.00710.2019
Different mechanisms for modulation of the initiation and steady-state of smooth pursuit eye movements
Abstract
Smooth pursuit eye movements are used by primates to track moving objects. They are initiated by sensory estimates of target speed represented in the middle temporal (MT) area of extrastriate visual cortex and then supported by motor feedback to maintain steady-state eye speed at target speed. Here, we show that reducing the coherence in a patch of dots for a tracking target degrades the eye speed both at the initiation of pursuit and during steady-state tracking, when eye speed reaches an asymptote well below target speed. The deficits are quantitatively different between the motor-supported steady-state of pursuit and the sensory-driven initiation of pursuit, suggesting separate mechanisms. The deficit in visually guided pursuit initiation could not explain the deficit in steady-state tracking. Pulses of target speed during steady-state tracking revealed lower sensitivities to image motion across the retina for lower values of dot coherence. However, sensitivity was not zero, implying that visual motion should still be driving eye velocity toward target velocity. When we changed dot coherence from 100% to lower values during accurate steady-state pursuit, we observed larger eye decelerations for lower coherences, as expected if motor feedback was reduced in gain. A simple pursuit model accounts for our data based on separate modulation of the strength of visual-motor transmission and motor feedback. We suggest that reduced dot coherence allows us to observe evidence for separate modulations of the gain of visual-motor transmission during pursuit initiation and of the motor corollary discharges that comprise eye velocity memory and support steady-state tracking.NEW & NOTEWORTHY We exploit low-coherence patches of dots to control the initiation and steady state of smooth pursuit eye movements and show that these two phases of movement are modulated separately by the reliability of visual motion signals. We conclude that the neural circuit for pursuit includes separate modulation of the strength of visual-motor transmission for movement initiation and of eye velocity positive feedback to support steady-state tracking.
Keywords: cerebellum; floccular complex; gain modulation; motion reliability; velocity memory; visual motion.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
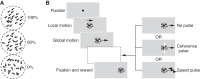
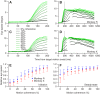
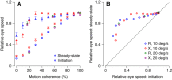


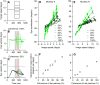

References
-
- Bakhtiari S, Pack CC. Functional specialization in the middle temporal area for smooth pursuit initiation [version 2; peer review: 2 approved]. MNI Open Res 2: 6, 2019. doi: 10.12688/mniopenres.12806.2. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

