Improvement in Patient-Reported Outcomes With Intensity-Modulated Radiotherapy (RT) Compared With Standard RT: A Report From the NRG Oncology RTOG 1203 Study
- PMID: 32073955
- PMCID: PMC7238486
- DOI: 10.1200/JCO.19.02381
Improvement in Patient-Reported Outcomes With Intensity-Modulated Radiotherapy (RT) Compared With Standard RT: A Report From the NRG Oncology RTOG 1203 Study
Abstract
Purpose: In oncology trials, the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) is the standard tool for reporting adverse events (AEs), but it may underreport symptoms experienced by patients. This analysis of the NRG Oncology RTOG 1203 compared symptom reporting by patients and clinicians during radiotherapy (RT).
Patients and methods: Patients with cervical or endometrial cancer requiring postoperative RT were randomly assigned to standard 4-field RT or intensity-modulated RT (IMRT). Patients completed the 6-item patient-reported outcomes version of the CTCAE (PRO-CTCAE) for GI toxicity assessing abdominal pain, diarrhea, and fecal incontinence at various time points. Patients reported symptoms on a 5-point scale. Clinicians recorded these AEs as CTCAE grades 1 to 5. Clinician- and patient-reported AEs were compared using McNemar's test for rates > 0%.
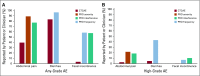
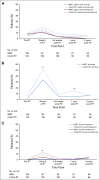
Results: Of 278 eligible patients, 234 consented and completed the PRO-CTCAE. Patients reported high-grade abdominal pain 19.1% (P < .0001), high-grade diarrhea 38.5% (P < .0001), and fecal incontinence 6.8% more frequently than clinicians. Similar effects were seen between grade ≥ 1 CTCAE toxicity and any-grade patient-reported toxicity. Between-arm comparison of patient-reported high-grade AEs revealed that at 5 weeks of RT, patients who received IMRT experienced fewer GI AEs than patients who received 4-field pelvic RT with regard to frequency of diarrhea (18.2% difference; P = .01), frequency of fecal incontinence (8.2% difference; P = .01), and interference of fecal incontinence (8.5% difference; P = .04).
Conclusion: Patient-reported AEs showed a reduction in symptoms with IMRT compared with standard RT, whereas clinician-reported AEs revealed no difference. Clinicians also underreported symptomatic GI AEs compared with patients. This suggests that patient-reported symptomatic AEs are important to assess in this disease setting.
Figures
Comment in
-
Radiation for Cancers of the Uterine Corpus and Cervix: Incremental Steps, and Glimmers of the Future.Int J Radiat Oncol Biol Phys. 2020 Nov 15;108(4):839-845. doi: 10.1016/j.ijrobp.2020.06.012. Int J Radiat Oncol Biol Phys. 2020. PMID: 33069345 No abstract available.
References
-
- Bruner DW, Hunt D, Michalski JM, et al. Preliminary patient-reported outcomes analysis of 3-dimensional radiation therapy versus intensity-modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group (RTOG) 0126 prostate cancer trial. Cancer. 2015;121:2422–2430. - PMC - PubMed
-
- Litwin MS, Lubeck DP, Henning JM, et al. Differences in urologist and patient assessments of health related quality of life in men with prostate cancer: Results of the CaPSURE database. J Urol. 1998;159:1988–1992. - PubMed
-
- Grossman SA, Sheidler VR, Swedeen K, et al. Correlation of patient and caregiver ratings of cancer pain. J Pain Symptom Manage. 1991;6:53–57. - PubMed
-
- Vogelzang NJ, Breitbart W, Cella D, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: Results of a tripart assessment survey—The Fatigue Coalition. Semin Hematol. 1997;34(suppl 2):4–12. - PubMed

