Validating indicators of CNS disorders in a swine model of neurological disease
- PMID: 32074109
- PMCID: PMC7029865
- DOI: 10.1371/journal.pone.0228222
Validating indicators of CNS disorders in a swine model of neurological disease
Abstract
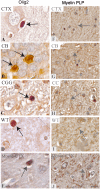
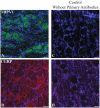
Genetically modified swine disease models are becoming increasingly important for studying molecular, physiological and pathological characteristics of human disorders. Given the limited history of these model systems, there remains a great need for proven molecular reagents in swine tissue. Here, to provide a resource for neurological models of disease, we validated antibodies by immunohistochemistry for use in examining central nervous system (CNS) markers in a recently developed miniswine model of neurofibromatosis type 1 (NF1). NF1 is an autosomal dominant tumor predisposition disorder stemming from mutations in NF1, a gene that encodes the Ras-GTPase activating protein neurofibromin. Patients classically present with benign neurofibromas throughout their bodies and can also present with neurological associated symptoms such as chronic pain, cognitive impairment, and behavioral abnormalities. As validated antibodies for immunohistochemistry applications are particularly difficult to find for swine models of neurological disease, we present immunostaining validation of antibodies implicated in glial inflammation (CD68), oligodendrocyte development (NG2, O4 and Olig2), and neuron differentiation and neurotransmission (doublecortin, GAD67, and tyrosine hydroxylase) by examining cellular localization and brain region specificity. Additionally, we confirm the utility of anti-GFAP, anti-Iba1, and anti-MBP antibodies, previously validated in swine, by testing their immunoreactivity across multiple brain regions in mutant NF1 samples. These immunostaining protocols for CNS markers provide a useful resource to the scientific community, furthering the utility of genetically modified miniswine for translational and clinical applications.
Conflict of interest statement
Our association with Sanford Research does not alter our adherence to PLOS ONE policies on sharing data and materials. The authors have declared that no competing interests exist. The specific roles of these authors are articulated in the ‘author contributions’ section.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

