ALK inhibitors for non-small cell lung cancer: A systematic review and network meta-analysis
- PMID: 32074131
- PMCID: PMC7029857
- DOI: 10.1371/journal.pone.0229179
ALK inhibitors for non-small cell lung cancer: A systematic review and network meta-analysis
Abstract
Background: We sought to assess the relative effects of individual anaplastic lymphoma kinase (ALK) inhibitors for the treatment of non-small cell lung cancer (NSCLC).
Methods: We searched MEDLINE, Embase, Cochrane CENTRAL, and grey literature (July 23, 2019) for randomized controlled trials (RCTs) that included participants with ALK- or ROS1-positive NSCLC who received any ALK inhibitor compared with placebo, another ALK inhibitor, or the same ALK inhibitor at a different dose. The primary outcome was treatment-related death. Secondary outcomes were overall survival (OS), progression-free survival (PFS), and serious adverse events. Data were pooled via meta-analysis and network meta-analysis, and risk of bias was assessed. PROSPERO: CRD42017077046.
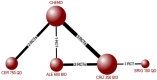
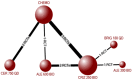
Results: Thirteen RCTs reporting outcomes of interest among participants with ALK-positive NSCLC were identified. Treatment-related deaths were rare, with 10 deaths attributed to crizotinib (risk difference v. chemotherapy: 0.49, 95% credible interval [CrI] -0.16 to 1.46; odds ratio 2.58 (0.76-11.37). All ALK inhibitors improved PSF relative to chemotherapy (hazard ratio [95% CrI]: crizotinib 0.46 [0.39-0.54]; ceritinib 0.52 [0.42-0.64]; alectinib 300 BID 0.16 [0.08-0.33]; alectinib 600 BID 0.23 [0.17-0.30]; brigatinib 0.23 [0.15-0.35]), while alectinib and brigatinib improved PFS over crizotinib and ceritinib (alectinib v. crizotinib 0.34 [0.17-0.70]; alectinib v. ceritinib 0.30 [0.14-0.64]; brigatinib v. crizotinib 0.49 [0.33-0.73]; brigatinib v. ceritinib 0.43 [0.27-0.70]). OS was improved with alectinib compared with chemotherapy (HR 0.57 [95% CrI 0.39-0.83]) and crizotinib (0.68 [0.48-0.96]). Use of crizotinib (odds ratio 2.08 [95% CrI 1.56-2.79]) and alectinib (1.60 [1.00-2.58]) but not ceritinib (1.25 [0.90-1.74), increased the risk of serious adverse events compared with chemotherapy. Results were generally consistent among treatment-experienced or naïve participants.
Conclusion(s): Treatment-related deaths were infrequent among ALK-positive NSCLC. PFS may be improved by alectinib and brigatinib relative to other ALK inhibitors; however, the assessment of OS is likely confounded by treatment crossover and should be interpreted with caution.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: BS is a paid information consultant/contractor to the Ottawa Hospital Heart Institute. DS has received personal fees from Roche Canada, Beohringer Ingelheim Canada, Novartis Canada, AstraZeneca Canada, Bristol-Myers Squibb Canada, Exactis, and Pfizer Canada, as well as grants from Celgene, outside of the submitted work. None declared by any other author. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Society CC. Lung cancer statistics 2017 [cited 2018 August 3]. http://www.cancer.ca/en/cancer-information/cancer-type/lung/statistics/?....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

