Presentation of BK polyomavirus-associated hemorrhagic cystitis after allogeneic hematopoietic cell transplantation
- PMID: 32074279
- PMCID: PMC7042995
- DOI: 10.1182/bloodadvances.2019000802
Presentation of BK polyomavirus-associated hemorrhagic cystitis after allogeneic hematopoietic cell transplantation
Abstract
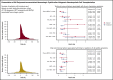
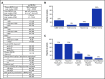
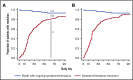
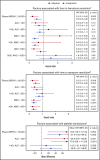
BK polyomavirus (BKPyV) has been associated with hemorrhagic cystitis (HC) after allogeneic hematopoietic cell transplantation (HCT), but the natural history of HC and factors associated with the clinical course are incompletely understood. We retrospectively analyzed allogeneic HCT patients transplanted from 2007-2017 who presented after platelet engraftment or after day 28 post-HCT with BKPyV-associated HC (BKPyV-HC), which was defined as a positive urine BKPyV PCR, ≥1 plasma BKPyV viral load result, and macroscopic hematuria (Bedi grade ≥2). Factors associated with resolution of macroscopic hematuria and resolution of all cystitis symptoms within 90 days after HC diagnosis were investigated in multivariable models. In 128 patients with BKPyV-HC, the median times from diagnosis to resolution of all symptoms, macroscopic hematuria, and urinary clots (present in 55% [71/128]) were 24 days (15-44), 17 days (10-30), and 14 days (5-26), respectively. Ninety percent of patients had BKPyV viremia at the onset of HC with a median viral load of 1850 copies/mL (interquartile range, 240-8550). In multivariable models, high plasma viral load (≥10 000 copies/mL) and cytopenias at the beginning of BKPyV-HC were significantly associated with longer macroscopic hematuria and cystitis symptoms. Use of cidofovir was not associated with shorter duration of illness. In conclusion, BKPyV-HC after allogeneic HCT is characterized by prolonged and severe symptoms and requires improved management strategies. High-grade viremia and cytopenias were associated with a longer duration of BKPyV-associated HC. Accurate descriptions of disease and factors associated with prolonged recovery will inform end points of future clinical trials.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: J.A.H. has served as a consultant for Nohla Therapeutics and Amplyx and has received research support from Nohla Therapeutics, Karius, and Takeda (formerly Shire), all unrelated to this research. S.A.P. has received research funding from Global Technologies and has participated in clinical trials with Chimerix. A.P.L. reports support in the form of research funding and as site investigator and consultancy for Merck, site investigator for Astellas, a member of the data monitoring committee for Novartis, a consultant and site investigator for Gilead, site investigator for Roche Diagnostics, site investigator for Hologic, and site investigator for Oxford Immunotech, all unrelated to this research. W.G.N. is employed by Chimerix. P.S.P. is an employee and shareholder of Vir Biotechnology. M.B. has served as consultant and received research funding from Chimerix and Vir Biotechnology. The remaining authors declare no competing financial interests.
Figures


References
-
- Egli A, Infanti L, Dumoulin A, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199(6):837-846. - PubMed
-
- Rorije NM, Shea MM, Satyanarayana G, et al. BK virus disease after allogeneic stem cell transplantation: a cohort analysis. Biol Blood Marrow Transplant. 2014;20(4):564-570. - PubMed
-
- Binet I, Nickeleit V, Hirsch HH. Polyomavirus infections in transplant recipients. Curr Opin Organ Transplant. 2000;5(3):210-216.
-
- Cesaro S, Dalianis T, Hanssen Rinaldo C, et al. ; ECIL-6 Group . ECIL guidelines for the prevention, diagnosis and treatment of BK polyomavirus-associated haemorrhagic cystitis in haematopoietic stem cell transplant recipients. J Antimicrob Chemother. 2018;73(1):12-21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

