Evaluation of Maternal, Embryo, and Placental Effects in CD-1 Mice following Gestational Exposure to Perfluorooctanoic Acid (PFOA) or Hexafluoropropylene Oxide Dimer Acid (HFPO-DA or GenX)
- PMID: 32074459
- PMCID: PMC7064328
- DOI: 10.1289/EHP6233
Evaluation of Maternal, Embryo, and Placental Effects in CD-1 Mice following Gestational Exposure to Perfluorooctanoic Acid (PFOA) or Hexafluoropropylene Oxide Dimer Acid (HFPO-DA or GenX)
Abstract
Background: Perfluorooctanoic acid (PFOA) is a poly- and perfluoroalkyl substance (PFAS) associated with adverse pregnancy outcomes in mice and humans, but little is known regarding one of its replacements, hexafluoropropylene oxide dimer acid (HFPO-DA, referred to here as GenX), both of which have been reported as contaminants in drinking water.
Objectives: We compared the toxicity of PFOA and GenX in pregnant mice and their developing embryo-placenta units, with a specific focus on the placenta as a hypothesized target.
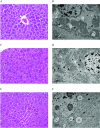
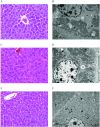
Methods: Pregnant CD-1 mice were exposed daily to PFOA (0, 1, or ) or GenX (0, 2, or ) via oral gavage from embryonic day (E) 1.5 to 11.5 or 17.5 to evaluate exposure effects on the dam and embryo-placenta unit. Gestational weight gain (GWG), maternal clinical chemistry, maternal liver histopathology, placental histopathology, embryo weight, placental weight, internal chemical dosimetry, and placental thyroid hormone levels were determined.
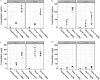
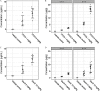
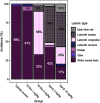
Results: Exposure to GenX or PFOA resulted in increased GWG, with increase in weight most prominent and of shortest latency with GenX exposure. Embryo weight was significantly lower after exposure to PFOA (9.4% decrease relative to controls). Effect sizes were similar for higher doses ( PFOA and GenX) and lower doses ( PFOA and GenX), including higher maternal liver weights, changes in liver histopathology, higher placental weights and embryo-placenta weight ratios, and greater incidence of placental abnormalities relative to controls. Histopathological features in placentas suggested that PFOA and GenX may exhibit divergent mechanisms of toxicity in the embryo-placenta unit, whereas PFOA- and GenX-exposed livers shared a similar constellation of adverse pathological features.
Conclusions: Gestational exposure to GenX recapitulated many documented effects of PFOA in CD-1 mice, regardless of its much shorter reported half-life; however, adverse effects toward the placenta appear to have compound-specific signatures. https://doi.org/10.1289/EHP6233.
Figures



References
-
- Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, et al. 2007. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect 115(11):1670–1676, PMID: 18008002, 10.1289/ehp.10334. - DOI - PMC - PubMed
-
- ATSDR (Agency for Toxic Substances and Disease Registry). 2019. PFAS exposure assessments. https://www.atsdr.cdc.gov/pfas/PFAS-Exposure-Assessments.html [accessed July 7, 2019].
-
- Ballesteros V, Costa O, Iñiguez C, Fletcher T, Ballester F, Lopez-Espinosa MJ. 2017. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: a systematic review of epidemiologic studies. Environ Int 99:15–28, PMID: 27884404, 10.1016/j.envint.2016.10.015. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
