Cytochrome c Oxidase Subunit 4 Isoform Exchange Results in Modulation of Oxygen Affinity
- PMID: 32075102
- PMCID: PMC7072730
- DOI: 10.3390/cells9020443
Cytochrome c Oxidase Subunit 4 Isoform Exchange Results in Modulation of Oxygen Affinity
Abstract
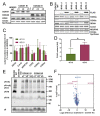
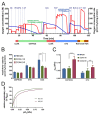
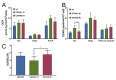
Cytochrome c oxidase (COX) is regulated through tissue-, development- or environment-controlled expression of subunit isoforms. The COX4 subunit is thought to optimize respiratory chain function according to oxygen-controlled expression of its isoforms COX4i1 and COX4i2. However, biochemical mechanisms of regulation by the two variants are only partly understood. We created an HEK293-based knock-out cellular model devoid of both isoforms (COX4i1/2 KO). Subsequent knock-in of COX4i1 or COX4i2 generated cells with exclusive expression of respective isoform. Both isoforms complemented the respiratory defect of COX4i1/2 KO. The content, composition, and incorporation of COX into supercomplexes were comparable in COX4i1- and COX4i2-expressing cells. Also, COX activity, cytochrome c affinity, and respiratory rates were undistinguishable in cells expressing either isoform. Analysis of energy metabolism and the redox state in intact cells uncovered modestly increased preference for mitochondrial ATP production, consistent with the increased NADH pool oxidation and lower ROS in COX4i2-expressing cells in normoxia. Most remarkable changes were uncovered in COX oxygen kinetics. The p50 (partial pressure of oxygen at half-maximal respiration) was increased twofold in COX4i2 versus COX4i1 cells, indicating decreased oxygen affinity of the COX4i2-containing enzyme. Our finding supports the key role of the COX4i2-containing enzyme in hypoxia-sensing pathways of energy metabolism.
Keywords: mitochondria, OXPHOS, respiratory chain, cytochrome c oxidase, COX, COX4 isoforms, COX4i2, oxygen affinity, p50, oxygen sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Huttemann M., Helling S., Sanderson T.H., Sinkler C., Samavati L., Mahapatra G., Varughese A., Lu G., Liu J., Ramzan R., et al. Regulation of mitochondrial respiration and apoptosis through cell signaling: Cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim. Biophys. Acta. 2012;1817:598–609. doi: 10.1016/j.bbabio.2011.07.001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

