Xenosiderophore Utilization Promotes Bacteroides thetaiotaomicron Resilience during Colitis
- PMID: 32075741
- PMCID: PMC7439322
- DOI: 10.1016/j.chom.2020.01.010
Xenosiderophore Utilization Promotes Bacteroides thetaiotaomicron Resilience during Colitis
Abstract
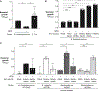
During short-lived perturbations, such as inflammation, the gut microbiota exhibits resilience and reverts to its original configuration. Although microbial access to the micronutrient iron is decreased during colitis, pathogens can scavenge iron by using siderophores. How commensal bacteria acquire iron during gut inflammation is incompletely understood. Curiously, the human commensal Bacteroides thetaiotaomicron does not produce siderophores but grows under iron-limiting conditions using enterobacterial siderophores. Using RNA-seq, we identify B. thetaiotaomicron genes that were upregulated during Salmonella-induced gut inflammation and were predicted to be involved in iron uptake. Mutants in the xusABC locus (BT2063-2065) were defective for xenosiderophore-mediated iron uptake in vitro. In the normal mouse gut, the XusABC system was dispensable, while a xusA mutant colonized poorly during colitis. This work identifies xenosiderophore utilization as a critical mechanism for B. thetaiotaomicron to sustain colonization during inflammation and suggests a mechanism of how interphylum iron metabolism contributes to gut microbiota resilience.
Keywords: bacteroidetes; gut inflammation; gut microbiota resilience; iron metabolism; siderophore.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The corresponding author (S.E.W.) is listed as an inventor on patent application WO2014200929A1, which describes a treatment to prevent the inflammation-associated expansion of Enterobacteriaceae. The other authors declare no competing interests.
Figures
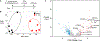
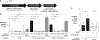
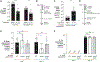

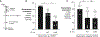

References
-
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, et al. (2013). Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 504(7480), 451–455. Published online 2013/11/15 DOI: 10.1038/nature12726. - DOI - PMC - PubMed
-
- Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, et al. (2012). Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 338(6103), 120–123. Published online 2012/08/21 DOI: 10.1126/science.1224820. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

