Glioma-Derived miRNA-Containing Extracellular Vesicles Induce Angiogenesis by Reprogramming Brain Endothelial Cells
- PMID: 32075753
- PMCID: PMC7148092
- DOI: 10.1016/j.celrep.2020.01.073
Glioma-Derived miRNA-Containing Extracellular Vesicles Induce Angiogenesis by Reprogramming Brain Endothelial Cells
Abstract
Glioblastoma (GBM) is characterized by aberrant vascularization and a complex tumor microenvironment. The failure of anti-angiogenic therapies suggests pathways of GBM neovascularization, possibly attributable to glioblastoma stem cells (GSCs) and their interplay with the tumor microenvironment. It has been established that GSC-derived extracellular vesicles (GSC-EVs) and their cargoes are proangiogenic in vitro. To further elucidate EV-mediated mechanisms of neovascularization in vitro, we perform RNA-seq and DNA methylation profiling of human brain endothelial cells exposed to GSC-EVs. To correlate these results to tumors in vivo, we perform histoepigenetic analysis of GBM molecular profiles in the TCGA collection. Remarkably, GSC-EVs and normal vascular growth factors stimulate highly distinct gene regulatory responses that converge on angiogenesis. The response to GSC-EVs shows a footprint of post-transcriptional gene silencing by EV-derived miRNAs. Our results provide insights into targetable angiogenesis pathways in GBM and miRNA candidates for liquid biopsy biomarkers.
Keywords: angiogenesis; biomarker; cancer stem cell; deconvolution; exRNA; extracellular vesicle; glioblastoma; miRNA; reprogramming; tumor microenvironment.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



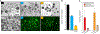
References
-
- Aslan C, Maralbashi S, Salari F, Kahroba H, Sigaroodi F, Kazemi T, and Kharaziha P. (2019). Tumor-derived exosomes: implication in angiogenesis and antiangiogenesis cancer therapy. J. Cell. Physiol 234, 16885–16903. - PubMed
-
- Bergers G, and Benjamin LE (2003). Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 3, 401–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

