Predicting clinical benefit of immunotherapy by antigenic or functional mutations affecting tumour immunogenicity
- PMID: 32075964
- PMCID: PMC7031381
- DOI: 10.1038/s41467-020-14562-z
Predicting clinical benefit of immunotherapy by antigenic or functional mutations affecting tumour immunogenicity
Abstract
Neoantigen burden is regarded as a fundamental determinant of response to immunotherapy. However, its predictive value remains in question because some tumours with high neoantigen load show resistance. Here, we investigate our patient cohort together with a public cohort by our algorithms for the modelling of peptide-MHC binding and inter-cohort genomic prediction of therapeutic resistance. We first attempt to predict MHC-binding peptides at high accuracy with convolutional neural networks. Our prediction outperforms previous methods in > 70% of test cases. We then develop a classifier that can predict resistance from functional mutations. The predictive genes are involved in immune response and EGFR signalling, whereas their mutation patterns reflect positive selection. When integrated with our neoantigen profiling, these anti-immunogenic mutations reveal higher predictive power than known resistance factors. Our results suggest that the clinical benefit of immunotherapy can be determined by neoantigens that induce immunity and functional mutations that facilitate immune evasion.
Conflict of interest statement
The authors declare no competing interests.
Figures
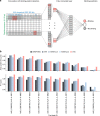
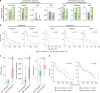
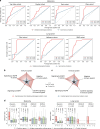


References
-
- The problem with neoantigen prediction. Nat. Biotechnol. 35, 97–97 (2017). https://www.ncbi.nlm.nih.gov/pubmed/28178261. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

